9 0 5 7 8
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 2.6 Breast Cancer (Dove Med Press)
- 3.9 Clin Epidemiol
- 3.3 Cancer Manag Res
- 3.9 Infect Drug Resist
- 3.6 Clin Interv Aging
- 4.8 Drug Des Dev Ther
- 2.8 Int J Chronic Obstr
- 8.0 Int J Nanomed
- 2.3 Int J Women's Health
- 3.2 Neuropsych Dis Treat
- 4.0 OncoTargets Ther
- 2.2 Patient Prefer Adher
- 2.8 Ther Clin Risk Manag
- 2.7 J Pain Res
- 3.3 Diabet Metab Synd Ob
- 4.3 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.9 Pharmgenomics Pers Med
- 3.5 Risk Manag Healthc Policy
- 4.5 J Inflamm Res
- 2.3 Int J Gen Med
- 4.1 J Hepatocell Carcinoma
- 3.2 J Asthma Allergy
- 2.3 Clin Cosmet Investig Dermatol
- 3.3 J Multidiscip Healthc

已发表论文
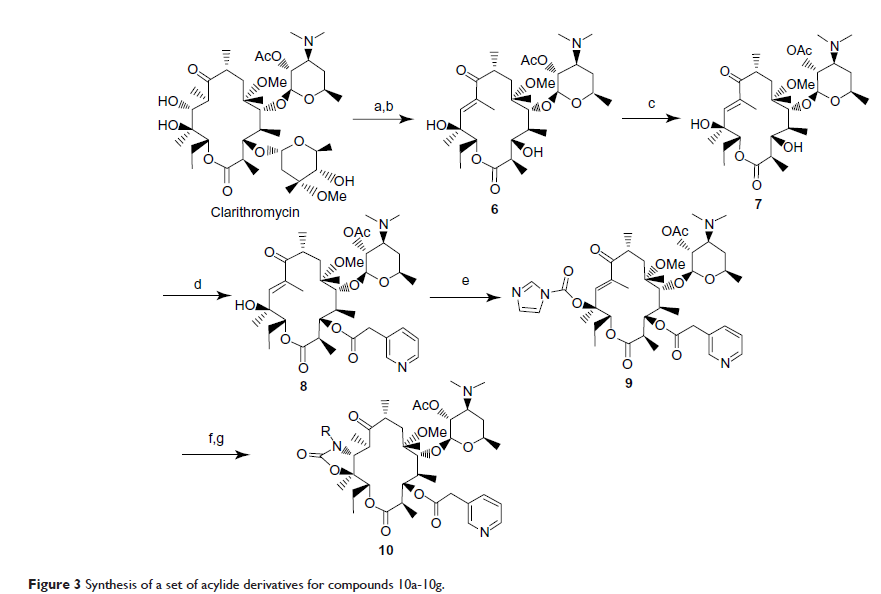
含一个芳基四唑基大环内酯类酰内酯衍生物合成与抗菌活性
Authors Shan LX, Sun PH, Guo BQ, Xu XJ, Li ZQ, Sun JZ, Zhou SF, Chen WM
Published Date September 2014 Volume 2014:8 Pages 1515—1525
DOI http://dx.doi.org/10.2147/DDDT.S65673
Received 7 April 2014, Accepted 21 May 2014, Published 24 September 2014
Abstract: Seventeen acylides bearing an aryl-tetrazolyl alkyl-substituted side chain were synthesized, starting from clarithromycin, via several reactions including hydrolysis, acetylating, esterification, carbamylation, and Michael addition. The structures of all new compounds were confirmed by 1H nuclear magnetic resonance spectroscopy, 13C nuclear magnetic resonance spectroscopy, and mass spectrometry. All these synthesized acylides were evaluated for in vitro antimicrobial activities against gram-positive pathogens (Staphylococcus aureus , Staphylococcus epidermidis ) and gram-negative pathogens (Pseudomonas aeruginosa , Escherichia coli ), using the broth microdilution method. Results showed that compounds 10e, 10f, 10g, 10h, 10o have good antibacterial activities.
Keywords: acylide, clarithromycin, synthesis, antibacterial activity
Keywords: acylide, clarithromycin, synthesis, antibacterial activity