9 0 6 7 6
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 2.6 Breast Cancer (Dove Med Press)
- 3.9 Clin Epidemiol
- 3.3 Cancer Manag Res
- 3.9 Infect Drug Resist
- 3.6 Clin Interv Aging
- 4.8 Drug Des Dev Ther
- 2.8 Int J Chronic Obstr
- 8.0 Int J Nanomed
- 2.3 Int J Women's Health
- 3.2 Neuropsych Dis Treat
- 4.0 OncoTargets Ther
- 2.2 Patient Prefer Adher
- 2.8 Ther Clin Risk Manag
- 2.7 J Pain Res
- 3.3 Diabet Metab Synd Ob
- 4.3 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.9 Pharmgenomics Pers Med
- 3.5 Risk Manag Healthc Policy
- 4.5 J Inflamm Res
- 2.3 Int J Gen Med
- 4.1 J Hepatocell Carcinoma
- 3.2 J Asthma Allergy
- 2.3 Clin Cosmet Investig Dermatol
- 3.3 J Multidiscip Healthc

使用新的 pH 响应性可激活的细胞穿透肽修饰的脂质体系统获得增强化的基于 siRNA 的癌症疗法
Authors Xiang B, Jia XL, Qi JL, Yang LP, Sun WH, Yan X, Yang SK, Cao DY, Du Q, Qi XR
Received 6 December 2016
Accepted for publication 28 February 2017
Published 28 March 2017 Volume 2017:12 Pages 2385—2405
DOI https://doi.org/10.2147/IJN.S129574
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Jiang Yang
Peer reviewer comments 2
Editor who approved publication: Dr Lei Yang
Abstract: As a potent therapeutic agent, small interfering RNA (siRNA)
has been exploited to silence critical genes involved in tumor initiation and
progression. However, development of a desirable delivery system is required to
overcome the unfavorable properties of siRNA such as its high degradability,
molecular size, and negative charge to help increase its accumulation in tumor
tissues and promote efficient cellular uptake and endosomal/lysosomal escape of
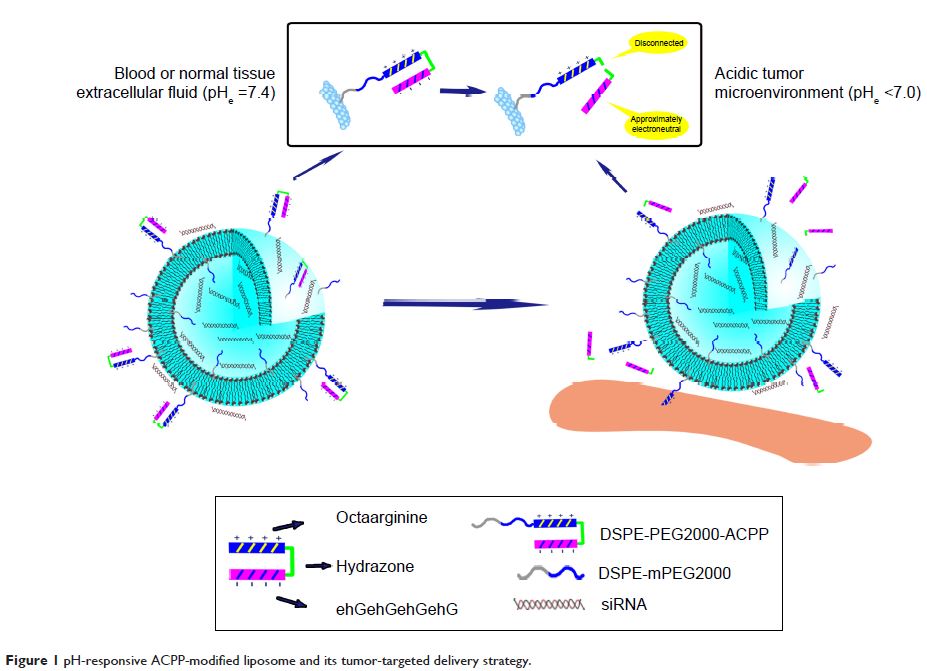
the nucleic acids. In this study, we developed a new activatable
cell-penetrating peptide (ACPP) that is responsive to an acidic tumor
microenvironment, which was then used to modify the surfaces of siRNA-loaded
liposomes. The ACPP is composed of a cell-penetrating peptide (CPP), an
acid-labile linker (hydrazone), and a polyanionic domain, including glutamic
acid and histidine. In the systemic circulation (pH 7.4), the surface
polycationic moieties of the CPP (polyarginine) are “shielded” by the
intramolecular electrostatic interaction of the inhibitory domain. When exposed
to a lower pH, a common property of solid tumors, the ACPP undergoes
acid-catalyzed breakage at the hydrazone site, and the consequent protonation
of histidine residues promotes detachment of the inhibitory peptide.
Subsequently, the unshielded CPP would facilitate the cellular membrane
penetration and efficient endosomal/lysosomal evasion of liposomal siRNA. A
series of investigations demonstrated that once exposed to an acidic pH, the
ACPP-modified liposomes showed elevated cellular uptake, downregulated
expression of polo-like kinase 1, and augmented cell apoptosis. In addition,
favorable siRNA avoidance of the endosome/lysosome was observed in both MCF-7
and A549 cells, followed by effective cytoplasmic release. In view of its acid
sensitivity and therapeutic potency, this newly developed pH-responsive and ACPP-mediated
liposome system represents a potential platform for siRNA-based cancer
treatment.
Keywords: siRNA,
ACPP, hydrazone, liposome, endosomal/lysosomal escape