9 0 6 7 6
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 2.6 Breast Cancer (Dove Med Press)
- 3.9 Clin Epidemiol
- 3.3 Cancer Manag Res
- 3.9 Infect Drug Resist
- 3.6 Clin Interv Aging
- 4.8 Drug Des Dev Ther
- 2.8 Int J Chronic Obstr
- 8.0 Int J Nanomed
- 2.3 Int J Women's Health
- 3.2 Neuropsych Dis Treat
- 4.0 OncoTargets Ther
- 2.2 Patient Prefer Adher
- 2.8 Ther Clin Risk Manag
- 2.7 J Pain Res
- 3.3 Diabet Metab Synd Ob
- 4.3 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.9 Pharmgenomics Pers Med
- 3.5 Risk Manag Healthc Policy
- 4.5 J Inflamm Res
- 2.3 Int J Gen Med
- 4.1 J Hepatocell Carcinoma
- 3.2 J Asthma Allergy
- 2.3 Clin Cosmet Investig Dermatol
- 3.3 J Multidiscip Healthc

Pembrolizumab 治疗实体癌的安全性和疗效特征:基于随机对照试验的合并再分析
Authors Wang MN, Ma XL, Guo LH, Xia F
Received 13 July 2017
Accepted for publication 9 August 2017
Published 27 September 2017 Volume 2017:11 Pages 2851—2860
DOI https://doi.org/10.2147/DDDT.S146286
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Akshita Wason
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Background: The aim of the present review is to systematically evaluate the
efficacy and safety of pembrolizumab by analyzing survival outcomes and at the
same time, to present evidence for future clinical applications of
anti-programmed cell death protein 1 (anti-PD-1) antibodies by analyzing the
efficacy and safety of pembrolizumab.
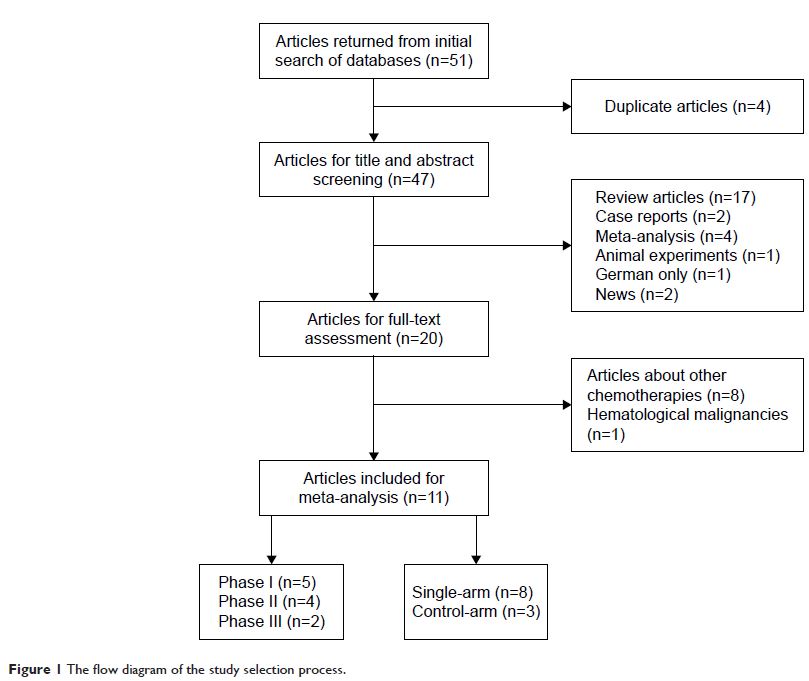
Methods: A comprehensive literature search of PubMed,
Medline, and Embase was performed for all relevant clinical trials. In this
study, adverse events of any grades and grades ≥3 were summarized and
calculated for event rates. For controlled trials, odd ratios (ORs) were
calculated to determine the role of pembrolizumab in adverse events. The
Kaplan–Meier survival curves were extracted for hazard ratio (HR) calculation
and survival outcomes were measured by progression-free survival (PFS).
Results: A total of 3,953 patients were included in
safety analyses. The results indicated that the overall incidence of any
treatment emergent adverse events was 74.3% (95% confidence interval [CI]:
0.671–0.805). The efficacy analysis involving 915 patients with advanced
melanoma suggested that 10 mg/kg of pembrolizumab every 3 weeks could
improve patients’ PFS (HR =0.73, 95% CI: 0.64–0.83).
Conclusion: Pembrolizumab is a promising therapeutic option that
could bring better survival outcomes but, at the same time, leads to higher
frequency of some adverse events.
Keywords: pembrolizumab,
safety, efficacy, meta-analysis