9 0 6 7 6
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 2.6 Breast Cancer (Dove Med Press)
- 3.9 Clin Epidemiol
- 3.3 Cancer Manag Res
- 3.9 Infect Drug Resist
- 3.6 Clin Interv Aging
- 4.8 Drug Des Dev Ther
- 2.8 Int J Chronic Obstr
- 8.0 Int J Nanomed
- 2.3 Int J Women's Health
- 3.2 Neuropsych Dis Treat
- 4.0 OncoTargets Ther
- 2.2 Patient Prefer Adher
- 2.8 Ther Clin Risk Manag
- 2.7 J Pain Res
- 3.3 Diabet Metab Synd Ob
- 4.3 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.9 Pharmgenomics Pers Med
- 3.5 Risk Manag Healthc Policy
- 4.5 J Inflamm Res
- 2.3 Int J Gen Med
- 4.1 J Hepatocell Carcinoma
- 3.2 J Asthma Allergy
- 2.3 Clin Cosmet Investig Dermatol
- 3.3 J Multidiscip Healthc

对实体瘤患者外周血循环内皮细胞及其亚群进行的优化的多参数流式细胞术分析:一项技术性分析
Authors Zhou F, Zhou Y, Yang M, Wen J, Dong J, Tan W
Received 22 November 2017
Accepted for publication 14 January 2018
Published 8 March 2018 Volume 2018:10 Pages 447—464
DOI https://doi.org/10.2147/CMAR.S157837
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Colin Mak
Peer reviewer comments 2
Editor who approved publication: Professor Kenan Onel
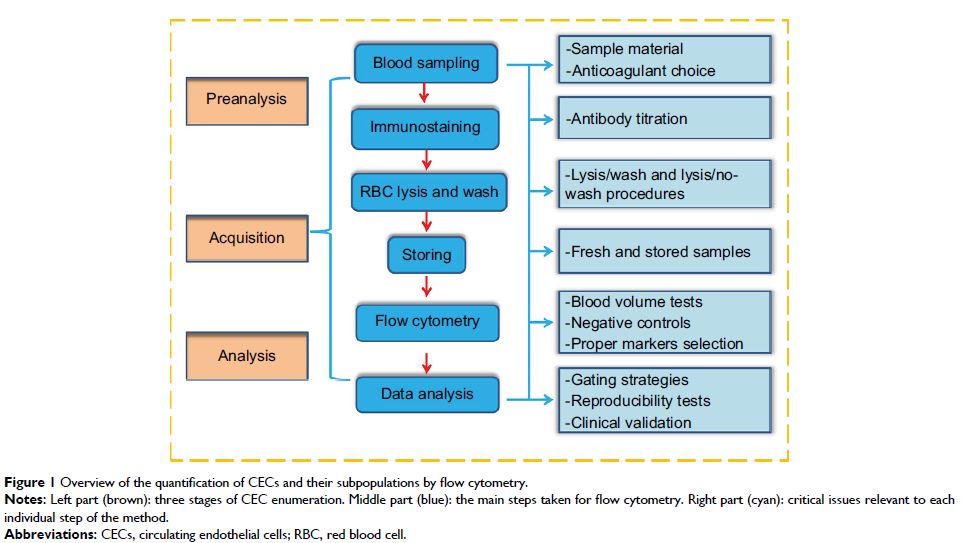
Background: Circulating endothelial cells (CECs) and their subpopulations
could be potential novel biomarkers for various malignancies. However, reliable
enumerable methods are warranted to further improve their clinical utility.
This study aimed to optimize a flow cytometric method (FCM) assay for CECs and
subpopulations in peripheral blood for patients with solid cancers.
Patients and
methods: An FCM assay was used to detect and
identify CECs. A panel of 60 blood samples, including 44 metastatic cancer
patients and 16 healthy controls, were used in this study. Some key issues of
CEC enumeration, including sample material and anticoagulant selection, optimal
titration of antibodies, lysis/wash procedures of blood sample preparation,
conditions of sample storage, sufficient cell events to enhance the signal,
fluorescence-minus-one controls instead of isotype controls to reduce
background noise, optimal selection of cell surface markers, and evaluating the
reproducibility of our method, were integrated and investigated. Wilcoxon and
Mann–Whitney U tests were used to determine
statistically significant differences.
Results: In this validation study, we refined a five-color FCM method to
detect CECs and their subpopulations in peripheral blood of patients with solid
tumors. Several key technical issues regarding preanalytical elements, FCM data
acquisition, and analysis were addressed. Furthermore, we clinically validated
the utility of our method. The baseline levels of mature CECs, endothelial
progenitor cells, and activated CECs were higher in cancer patients than
healthy subjects (P <0.01). However, there was no
significant difference in resting CEC levels between healthy subjects and
cancer patients (P =0.193).
Conclusion: We integrated and comprehensively addressed significant technical
issues found in previously published assays and validated the reproducibility
and sensitivity of our proposed method. Future work is required to explore the
potential of our optimized method in clinical oncologic applications.
Keywords: circulating endothelial cells, CECs, CEC subpopulations, flow
cytometry, methods