9 0 5 7 8
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 2.6 Breast Cancer (Dove Med Press)
- 3.9 Clin Epidemiol
- 3.3 Cancer Manag Res
- 3.9 Infect Drug Resist
- 3.6 Clin Interv Aging
- 4.8 Drug Des Dev Ther
- 2.8 Int J Chronic Obstr
- 8.0 Int J Nanomed
- 2.3 Int J Women's Health
- 3.2 Neuropsych Dis Treat
- 4.0 OncoTargets Ther
- 2.2 Patient Prefer Adher
- 2.8 Ther Clin Risk Manag
- 2.7 J Pain Res
- 3.3 Diabet Metab Synd Ob
- 4.3 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.9 Pharmgenomics Pers Med
- 3.5 Risk Manag Healthc Policy
- 4.5 J Inflamm Res
- 2.3 Int J Gen Med
- 4.1 J Hepatocell Carcinoma
- 3.2 J Asthma Allergy
- 2.3 Clin Cosmet Investig Dermatol
- 3.3 J Multidiscip Healthc

在癌症患者中,ipilimumab 加 GM-CSF 与单用 ipilimumab 治疗效果和安全性的比较 一项对结局的综合分析
Authors Chen P, Chen FC, Zhou BH
Received 16 October 2017
Accepted for publication 11 April 2018
Published 4 July 2018 Volume 2018:12 Pages 2025—2038
DOI https://doi.org/10.2147/DDDT.S154258
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Cristina Weinberg
Peer reviewer comments 3
Editor who approved publication: Dr Sukesh Voruganti
Background: Recent clinical studies have shown that initial therapy with
combined cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) blockade and
granulocyte-macrophage colony-stimulating factor (GM-CSF)-based immunotherapies
can enhance the antitumor efficacy of this approach. A key unanswered question
is whether systemic GM-CSF enhances CTLA-4 blockade. Thus, the objective of
this study was taking a meta-analysis of randomized controlled trials to
compare the effect of ipilimumab plus GM-CSF versus ipilimumab alone on overall
response, overall survival, and progression-free survival, as well as the risk
of adverse events (AEs) in patients with cancer.
Materials and
methods: Searches were made in electronic
databases PubMed and Embase, and conference abstracts published by the American
Society of Clinical Oncology from 2000 to 2017. Statistical analyses were
carried out using either random-effects or fixed-effects models according to
the heterogeneity of eligible studies.
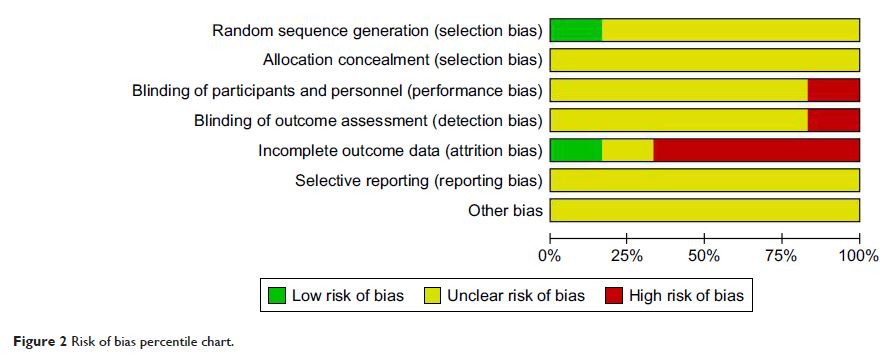
Results: Six trials comprising of 445 patients were included in the
meta-analysis. Combination group was superior to the ipilimumab alone in
overall response rate, progression-free survival, and overall survival rate
(combined relative risk [RR]=1.34, 95% CI: 1.24–1.45, P =0.09; combined hazard ratio
[HR]=0.57, 95% CI: 0.32–1.02, P =0.06; combined
HR=0.70, 95% CI: 0.60–0.82, P <0.001).
Patients with combination therapies had a lower incidence of AEs including
high-grade diarrhea (combined RR=0.27, 95% CI: 0.11–0.70, P =0.007), nausea (combined
RR=0.25, 95% CI: 0.07–0.89, P =0.03), colitis
(combined RR=0.34, 95% CI: 0.13–0.86, P =0.02), and
fatigue (combined RR=0.91, 95% CI: 0.37–2.2.3, P =0.84)
compared to the group having ipilimumab alone.
Conclusion: These data suggested that the combination of ipilimumab and GM-CSF
was associated with a significant improvement in overall survival and lower
high-grade toxicities, but there is no difference in overall response rate and
progression-free survival among the cancer patients. Therefore, large-scale and
well-designed studies are needed to summarize and analyze the data to draw a
more convincing conclusion.
Keywords: ipilimumab, sargramostim, efficacy, safety, survival,
meta-analysis