9 0 6 7 6
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 2.6 Breast Cancer (Dove Med Press)
- 3.9 Clin Epidemiol
- 3.3 Cancer Manag Res
- 3.9 Infect Drug Resist
- 3.6 Clin Interv Aging
- 4.8 Drug Des Dev Ther
- 2.8 Int J Chronic Obstr
- 8.0 Int J Nanomed
- 2.3 Int J Women's Health
- 3.2 Neuropsych Dis Treat
- 4.0 OncoTargets Ther
- 2.2 Patient Prefer Adher
- 2.8 Ther Clin Risk Manag
- 2.7 J Pain Res
- 3.3 Diabet Metab Synd Ob
- 4.3 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.9 Pharmgenomics Pers Med
- 3.5 Risk Manag Healthc Policy
- 4.5 J Inflamm Res
- 2.3 Int J Gen Med
- 4.1 J Hepatocell Carcinoma
- 3.2 J Asthma Allergy
- 2.3 Clin Cosmet Investig Dermatol
- 3.3 J Multidiscip Healthc

纳米结构钛通过影响成骨环境中的巨噬细胞极化来调节骨整合
Authors Wang J, Meng F, Song W, Jin J, Ma Q, Fei D, Fang L, Chen L, Wang Q, Zhang Y
Received 28 January 2018
Accepted for publication 24 April 2018
Published 10 July 2018 Volume 2018:13 Pages 4029—4043
DOI https://doi.org/10.2147/IJN.S163956
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Alexander Kharlamov
Peer reviewer comments 2
Editor who approved publication: Dr Linlin Sun
Introduction: Fabricating nanostructured surface topography represents the mainstream
approach to induce osteogenesis for the next-generation bone implant. In the
past, the bone implant was designed to minimize host repulsive reactions in
order to acquire biocompatibility. However, increasing reports indicate that
the absence of an appropriate immune response cannot acquire adequate
osseointegration after implantation in vivo.
Materials and methods: We prepared different topographies on the
surface of titanium (Ti) specimens by grinding, etching and anodizing, and they
were marked as polished specimen (P), specimen with nanotubes (NTs) in small
diameters (NT-30) and specimen with NTs in large diameters (NT-100). We
evaluated the ability of different topographies of the specimen to induce
osteogenic differentiation of mice bone marrow mesenchymal stem cells (BMSCs)
in vitro and to induce osseointegration in vivo. Furthermore, we investigated
the effect of different topographies on the polarization and secretion of
macrophages, and the effect of macrophage polarization on topography-induced
osteogenic differentiation of mice BMSCs. Finally, we verified the effect of
macrophage polarization on topography-induced osseointegration in vivo by using
Cre*RBP-Jfl/fl mice in which classically activated
macrophage was restrained.
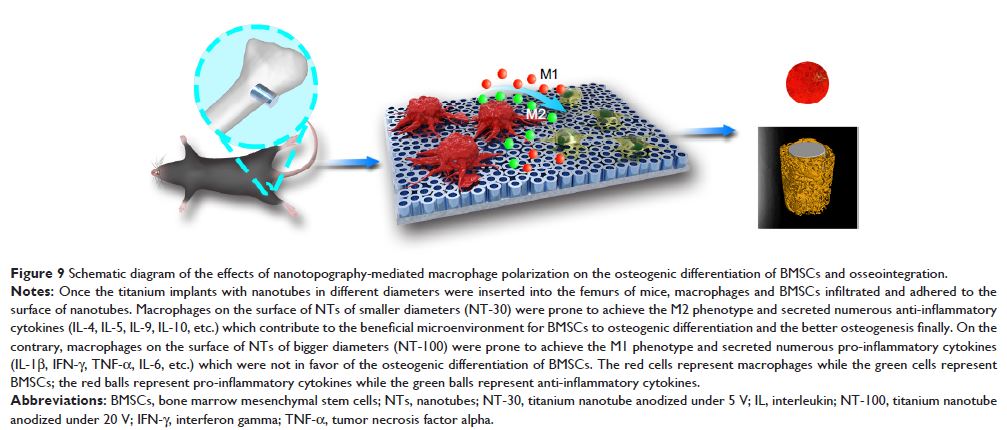
Results: The osteogenic differentiation of mice BMSCs induced
by specimen with different topographies was NT-100>NT-30>P, while the
osseointegration induced by specimen with different topographies in vivo was
NT-30>NT-100>P. In addition, specimen of NT-30 could induce more
macrophages to M2 polarization, while specimen of P and NT-100 could induce
more macrophages to M1 polarization. When co-culture mice BMSCs and macrophages
on specimen with different topographies, the osteogenic differentiation of mice
BMSCs was NT-30>NT-100≥P. The osseointegration induced by NT-100 in
Cre*RBP-Jfl/fl mice was much better than that of wild
type mice.
Conclusion: It
is suggested that the intrinsic immunomodulatory effects of nanomaterials are
not only crucial to evaluate the in vivo biocompatibility but also required to
determine the final osseointegration. To clarify the immune response and
osseointegration may be beneficial for the designation and optimization of the
bone implant.
Keywords: nanomaterials,
topography, immunomodulatory effects, macrophage polarization, osseointegration