9 0 5 7 8
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 2.6 Breast Cancer (Dove Med Press)
- 3.9 Clin Epidemiol
- 3.3 Cancer Manag Res
- 3.9 Infect Drug Resist
- 3.6 Clin Interv Aging
- 4.8 Drug Des Dev Ther
- 2.8 Int J Chronic Obstr
- 8.0 Int J Nanomed
- 2.3 Int J Women's Health
- 3.2 Neuropsych Dis Treat
- 4.0 OncoTargets Ther
- 2.2 Patient Prefer Adher
- 2.8 Ther Clin Risk Manag
- 2.7 J Pain Res
- 3.3 Diabet Metab Synd Ob
- 4.3 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.9 Pharmgenomics Pers Med
- 3.5 Risk Manag Healthc Policy
- 4.5 J Inflamm Res
- 2.3 Int J Gen Med
- 4.1 J Hepatocell Carcinoma
- 3.2 J Asthma Allergy
- 2.3 Clin Cosmet Investig Dermatol
- 3.3 J Multidiscip Healthc

已发表论文
对精神分裂症初发患者使用帕潘立酮棕榈酸盐的疗效、安全性和对入院治疗的影响
Authors Zhang F, Si T, Chiou CF, Harris AWF, Kim CY, Jahagirdar P, Ascher S
Published Date March 2015 Volume 2015:11 Pages 657—668
DOI http://dx.doi.org/10.2147/NDT.S77778
Received 19 November 2014, Accepted 19 December 2014, Published 11 March 2015
Approved for publication by Professor Wai Kwong Tang
Objective: To evaluate the efficacy, safety, and impact on hospitalizations of long-acting injectable paliperidone palmitate (PP) treatment, in patients with recent-onset schizophrenia who had not responded satisfactorily to oral antipsychotics.
Methods: In this 18-month, open-label, Phase-IIIb study from Asia-Pacific region, patients (18–50 years) with recent-onset (≤5 years) schizophrenia unsatisfactorily treated with previous oral antipsychotics were initiated on PP 150 mg eq on day 1, 100 mg eq on day 8, followed by flexible once monthly maintenance doses of 50–150 mg eq. The number and duration of hospitalizations were compared using a mirror analysis method between two periods: retrospective (12 months before PP initiation) and prospective (12 and 18 months after PP treatment) periods.
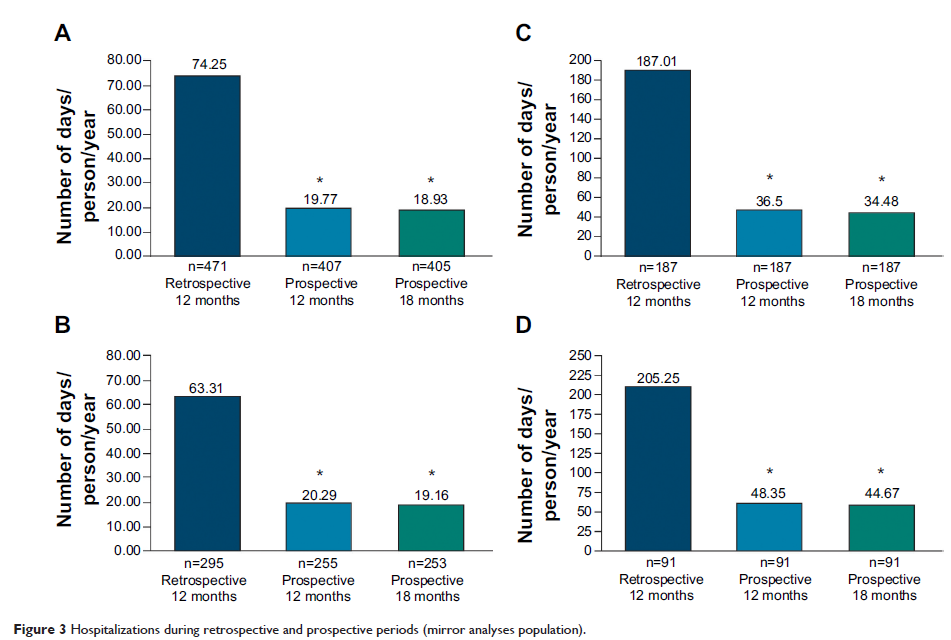
Results: A total of 303 out of 521 (58%) patients (mean age, 28.7 years; 65.5% men, 92.5% Asian) completed the study. Positive and Negative Syndrome Scale (PANSS) total score improved significantly from baseline to month 18 (mean [standard deviation, SD] change: -11.3 [21.38], P <0.0001, primary endpoint). Subgroup analysis revealed greater improvements among patients with worse disease severity at baseline: PANSS ≥70 versus <70 (mean [SD] change: -23.1 [24.62] vs -4.7 [15.98], P <0.0001 each). Secondary efficacy endpoints such as Clinical Global Impression of Schizophrenia (CGI-SCH), Medication Satisfaction Questionnaire (MSQ) scores showed significant improvements (P <0.0001) from baseline; 33.3% patients achieved symptom remission. In mirror analyses set (N=474), PP significantly (P <0.0001) reduced mean number of hospitalization days/person/year (12-month: 74.3 vs 19.7; 18-month: 74.3 vs 18.9) as well as percentage of patients requiring hospitalization in past 12 months (12-month: 39.7% vs 24.6%; 18-month: 39.7% vs 25%), and PP treatment increased the proportion of patients not requiring hospitalization (12-month: 60.3% vs 75.4%; 18-month: 60.3% vs 75%) from retrospective to prospective period. Adverse events (≥15%) were extrapyramidal symptoms-related (31.3%), injection-site pain (18.6%), and insomnia (15.2%).
Conclusion: PP was efficacious and generally tolerable with significant reductions observed in both number of hospitalizations and days spent in hospital.
Trial registration number: ClinicalTrials.gov: NCT01051531.
Keywords: atypical antipsychotics, long-acting injectables, open-label, paliperidone palmitate, schizophrenia
Methods: In this 18-month, open-label, Phase-IIIb study from Asia-Pacific region, patients (18–50 years) with recent-onset (≤5 years) schizophrenia unsatisfactorily treated with previous oral antipsychotics were initiated on PP 150 mg eq on day 1, 100 mg eq on day 8, followed by flexible once monthly maintenance doses of 50–150 mg eq. The number and duration of hospitalizations were compared using a mirror analysis method between two periods: retrospective (12 months before PP initiation) and prospective (12 and 18 months after PP treatment) periods.
Results: A total of 303 out of 521 (58%) patients (mean age, 28.7 years; 65.5% men, 92.5% Asian) completed the study. Positive and Negative Syndrome Scale (PANSS) total score improved significantly from baseline to month 18 (mean [standard deviation, SD] change: -11.3 [21.38], P <0.0001, primary endpoint). Subgroup analysis revealed greater improvements among patients with worse disease severity at baseline: PANSS ≥70 versus <70 (mean [SD] change: -23.1 [24.62] vs -4.7 [15.98], P <0.0001 each). Secondary efficacy endpoints such as Clinical Global Impression of Schizophrenia (CGI-SCH), Medication Satisfaction Questionnaire (MSQ) scores showed significant improvements (P <0.0001) from baseline; 33.3% patients achieved symptom remission. In mirror analyses set (N=474), PP significantly (P <0.0001) reduced mean number of hospitalization days/person/year (12-month: 74.3 vs 19.7; 18-month: 74.3 vs 18.9) as well as percentage of patients requiring hospitalization in past 12 months (12-month: 39.7% vs 24.6%; 18-month: 39.7% vs 25%), and PP treatment increased the proportion of patients not requiring hospitalization (12-month: 60.3% vs 75.4%; 18-month: 60.3% vs 75%) from retrospective to prospective period. Adverse events (≥15%) were extrapyramidal symptoms-related (31.3%), injection-site pain (18.6%), and insomnia (15.2%).
Conclusion: PP was efficacious and generally tolerable with significant reductions observed in both number of hospitalizations and days spent in hospital.
Trial registration number: ClinicalTrials.gov: NCT01051531.
Keywords: atypical antipsychotics, long-acting injectables, open-label, paliperidone palmitate, schizophrenia