9 0 5 7 8
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 2.6 Breast Cancer (Dove Med Press)
- 3.9 Clin Epidemiol
- 3.3 Cancer Manag Res
- 3.9 Infect Drug Resist
- 3.6 Clin Interv Aging
- 4.8 Drug Des Dev Ther
- 2.8 Int J Chronic Obstr
- 8.0 Int J Nanomed
- 2.3 Int J Women's Health
- 3.2 Neuropsych Dis Treat
- 4.0 OncoTargets Ther
- 2.2 Patient Prefer Adher
- 2.8 Ther Clin Risk Manag
- 2.7 J Pain Res
- 3.3 Diabet Metab Synd Ob
- 4.3 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.9 Pharmgenomics Pers Med
- 3.5 Risk Manag Healthc Policy
- 4.5 J Inflamm Res
- 2.3 Int J Gen Med
- 4.1 J Hepatocell Carcinoma
- 3.2 J Asthma Allergy
- 2.3 Clin Cosmet Investig Dermatol
- 3.3 J Multidiscip Healthc

奥拉帕尼(olaparib)在癌症治疗中的疗效和安全性:对随机对照试验的一项综合分析
Authors Guo XX, Wu HL, Shi HY, Su L, Zhang X
Received 30 March 2018
Accepted for publication 12 June 2018
Published 10 August 2018 Volume 2018:10 Pages 2553—2562
DOI https://doi.org/10.2147/CMAR.S169558
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Andrew Yee
Peer reviewer comments 4
Editor who approved publication: Dr Antonella D'Anneo
Purpose: PARP inhibition is an exciting new anticancer strategy. As the
first PARP inhibitor approved for the treatment of advanced BRCA -mutated ovarian cancer,
olaparib has proven to be effective in the treatment of several solid tumors.
We performed a meta-analysis of published randomized controlled trials to
evaluate the efficacy and safety of olaparib in cancer patients.
Methods: PubMed, Embase, and oncology-conference proceedings were searched
for relevant studies. End points were overall survival (OS), progression-free
survival (PFS), overall response rate (ORR), and grade 3/4 adverse events.
Pooled hazard ratio (HR)/risk ratio (RR) and 95% CI were calculated using
random or fixed-effect models.
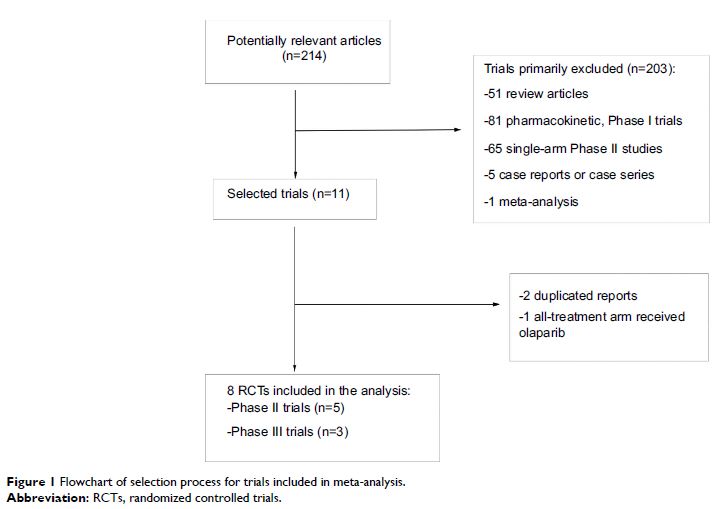
Results: Eight trials involving 1,957 patients were ultimately identified. The
pooled analysis demonstrated that olaparib treatment significantly improved PFS
(HR 0.62, 95% CI 0.47–0.82; P =0.001), OS (HR
0.82, 95% CI 0.73–0.93; P =0.001), and ORR
(RR 1.38, 95% CI 1.16–1.65; P <0.001) when
compared with therapy not containing olaparib. This association was further
confirmed by sensitivity analysis. Additionally, olaparib treatment offered a
significant survival benefit for patients with BRCA mutation.
Moreover, treatment with olaparib was associated with a significant increase in
risk of severe anemia.
Conclusion: Olaparib treatment has better treatment response compared with therapy
not containing olaparib, whereas olaparib can increase the risk of severe
anemia.
Keywords: olaparib, efficacy, safety, cancers, meta-analysis, RCTs