9 0 5 7 8
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 2.6 Breast Cancer (Dove Med Press)
- 3.9 Clin Epidemiol
- 3.3 Cancer Manag Res
- 3.9 Infect Drug Resist
- 3.6 Clin Interv Aging
- 4.8 Drug Des Dev Ther
- 2.8 Int J Chronic Obstr
- 8.0 Int J Nanomed
- 2.3 Int J Women's Health
- 3.2 Neuropsych Dis Treat
- 4.0 OncoTargets Ther
- 2.2 Patient Prefer Adher
- 2.8 Ther Clin Risk Manag
- 2.7 J Pain Res
- 3.3 Diabet Metab Synd Ob
- 4.3 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.9 Pharmgenomics Pers Med
- 3.5 Risk Manag Healthc Policy
- 4.5 J Inflamm Res
- 2.3 Int J Gen Med
- 4.1 J Hepatocell Carcinoma
- 3.2 J Asthma Allergy
- 2.3 Clin Cosmet Investig Dermatol
- 3.3 J Multidiscip Healthc

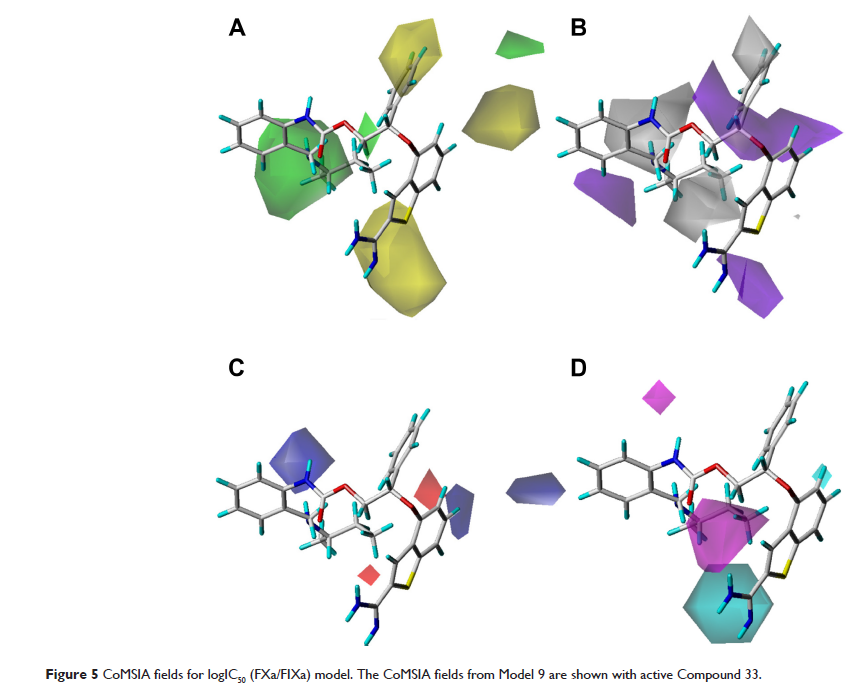
通过氨基苯并噻吩基衍生物的三维定量结构 - 性能关系进行新的选择性凝血因子 IXa 抑制剂的设计和预测
Authors Gao JS, Tong XP, Chang YQ, He YX, Mei YD, Tan PH, Guo JL, Liao GC, Xiao GK, Chen WM, Zhou SF, Sun PH
Published Date March 2015 Volume 2015:9 Pages 1743—1759
DOI http://dx.doi.org/10.2147/DDDT.S75282
Received 2 October 2014, Accepted 17 December 2014, Published 23 March 2015
Abstract: Factor IXa
(FIXa), a blood coagulation factor, is specifically inhibited at the initiation
stage of the coagulation cascade, promising an excellent approach for
developing selective and safe anticoagulants. Eighty-four amidinobenzothiophene
antithrombotic derivatives targeting FIXa were selected to establish
three-dimensional quantitative structure–activity relationship (3D-QSAR) and
three-dimensional quantitative structure–selectivity relationship (3D-QSSR)
models using comparative molecular field analysis and comparative similarity
indices analysis methods. Internal and external cross-validation techniques
were investigated as well as region focusing and bootstrapping. The
satisfactory q 2 values of 0.753 and
0.770, and r 2 values of
0.940 and 0.965 for 3D-QSAR and 3D-QSSR, respectively, indicated that the
models are available to predict both the inhibitory activity and selectivity on
FIXa against Factor Xa, the activated status of Factor X. This work revealed
that the steric, hydrophobic, and H-bond factors should appropriately be taken
into account in future rational design, especially the modifications at the
2'-position of the benzene and the 6-position of the benzothiophene in the R
group, providing helpful clues to design more active and selective FIXa
inhibitors for the treatment of thrombosis. On the basis of the
three-dimensional quantitative structure–property relationships, 16 new
potent molecules have been designed and are predicted to be more active and
selective than Compound 33, which has the best activity as reported in the
literature.
Keywords: CoMFA, CoMSIA,
3D-QSAR, 3D-QSSR, benzothiophene antithrombosis