9 0 6 7 6
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 2.6 Breast Cancer (Dove Med Press)
- 3.9 Clin Epidemiol
- 3.3 Cancer Manag Res
- 3.9 Infect Drug Resist
- 3.6 Clin Interv Aging
- 4.8 Drug Des Dev Ther
- 2.8 Int J Chronic Obstr
- 8.0 Int J Nanomed
- 2.3 Int J Women's Health
- 3.2 Neuropsych Dis Treat
- 4.0 OncoTargets Ther
- 2.2 Patient Prefer Adher
- 2.8 Ther Clin Risk Manag
- 2.7 J Pain Res
- 3.3 Diabet Metab Synd Ob
- 4.3 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.9 Pharmgenomics Pers Med
- 3.5 Risk Manag Healthc Policy
- 4.5 J Inflamm Res
- 2.3 Int J Gen Med
- 4.1 J Hepatocell Carcinoma
- 3.2 J Asthma Allergy
- 2.3 Clin Cosmet Investig Dermatol
- 3.3 J Multidiscip Healthc

血浆 ESR1 突变对雌激素受体阳性乳腺癌晚期内分泌治疗疗效的预测能力
Authors Du YF, Li N, Jiao X, Li K, Yan SC
Received 18 April 2018
Accepted for publication 26 June 2018
Published 19 September 2018 Volume 2018:11 Pages 6023—6029
DOI https://doi.org/10.2147/OTT.S171465
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Amy Norman
Peer reviewer comments 2
Editor who approved publication: Dr XuYu Yang
Purpose: The predictive ability of plasma ESR1 mutations
for outcomes among patients with advanced breast cancer undergoing endocrine
therapy (ET) remains disputable. We performed a comprehensive meta-analysis of
published studies to clarify the impact of plasma ESR1 mutations on clinical
outcomes for patients after subsequent ET.
Materials and
methods: An electronic search was performed
to identify eligible studies. Studies analyzing progression-free survival (PFS)
and/or overall survival (OS) according to plasma ESR1 mutation status after
subsequent ET were included. HRs were calculated using a fixed- or
random-effects model according to heterogeneity. Pooled HRs and 95% CIs were
used to estimate the effects.
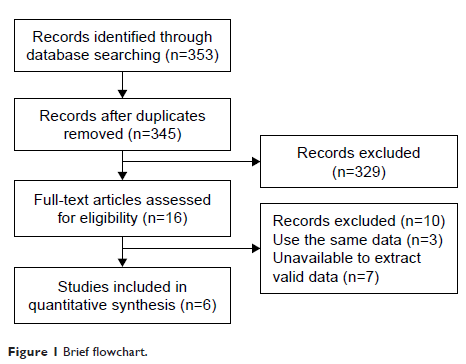
Results: Six studies including 705 patients with advanced breast cancer met
the inclusion criteria. The impact of plasma ESR1 mutations
on PFS and OS after subsequent ET was reported in six studies (seven groups)
and two studies, respectively. Meta-analysis results showed that the pooled HR
for ESR1 mutations was 1.70 (95%
CI, 1.05–2.74; P =0.03) for OS,
which was statistically significant for predicting poor survival, and 1.56 (95%
CI, 1.13–2.14; P =0.006) for PFS;
however, Begg’s and Egger’s test results identified the presence of bias. The
trim-and-fill method was used, and after incorporation of the imputed studies,
the HR was 1.16 (95% CI, 0.88–1.53, P =0.30) for PFS,
which indicates that plasma ESR1 mutation
had no effect on PFS after subsequent ET. Subgroup analysis suggested that
plasma ESR1 mutations were
correlated with shorter PFS (HR, 1.98; 95% CI, 1.12–3.51; P =0.02) in patients
subsequently treated with aromatase inhibitors (AIs), whereas no association
with PFS was observed for patients subsequently treated with non-AI ET (HR,
1.08; 95% CI, 0.85–1.38; P =0.54) or
fulvestrant (HR, 1.03; 95% CI, 0.79–1.34; P =0.83).
Conclusion: The current meta-analysis demonstrates that plasma ESR1 mutation status is not a
predictor of ET efficacy for all drugs without distinction in patients with
hormone-receptor-positive advanced breast cancer. ESR1 mutation predicted a
poor response to AIs, whereas it was not predictive of non-AI ET efficacy,
especially for fulvestrant.
Keywords: ESR1 mutation, breast cancer,
endocrine therapy, efficacy predictor