9 0 6 7 6
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 2.6 Breast Cancer (Dove Med Press)
- 3.9 Clin Epidemiol
- 3.3 Cancer Manag Res
- 3.9 Infect Drug Resist
- 3.6 Clin Interv Aging
- 4.8 Drug Des Dev Ther
- 2.8 Int J Chronic Obstr
- 8.0 Int J Nanomed
- 2.3 Int J Women's Health
- 3.2 Neuropsych Dis Treat
- 4.0 OncoTargets Ther
- 2.2 Patient Prefer Adher
- 2.8 Ther Clin Risk Manag
- 2.7 J Pain Res
- 3.3 Diabet Metab Synd Ob
- 4.3 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.9 Pharmgenomics Pers Med
- 3.5 Risk Manag Healthc Policy
- 4.5 J Inflamm Res
- 2.3 Int J Gen Med
- 4.1 J Hepatocell Carcinoma
- 3.2 J Asthma Allergy
- 2.3 Clin Cosmet Investig Dermatol
- 3.3 J Multidiscip Healthc

与晚期非小细胞肺癌的免疫检查点抑制剂相关联的完全反应:对 9 项随机对照试验的综合分析
Authors Li J, He Q, Yu X, Khan K, Weng X, Guan M
Received 24 September 2018
Accepted for publication 3 December 2018
Published 18 February 2019 Volume 2019:11 Pages 1623—1629
DOI https://doi.org/10.2147/CMAR.S188551
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Cristina Weinberg
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Purpose: The
purposes of this study were to investigate whether the use of immune checkpoint
inhibitors (ICIs) in advanced non-small-cell lung cancer (NSCLC) would increase
the possibility of archiving complete response (CR) and assess the surrogate
end points for overall survival (OS).
Methods: We
calculated the incidence and relative risk (RR) of CR events in patients
assigned to ICIs compared to that in controls. Simple linear regression models
were fitted for median OS and each surrogate (median progression-free survival
[PFS], CRs, and objective response rate [ORR]).
Results: A total
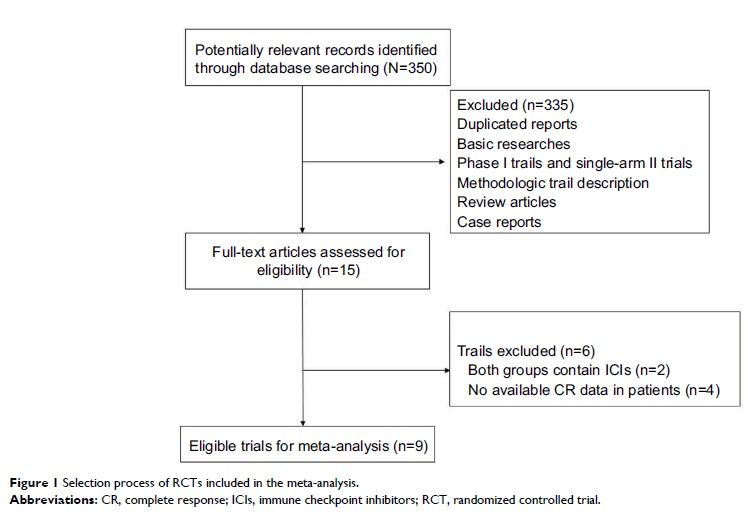
of 4,803 NSCLC patients from nine randomized controlled trials (RCTs) were
included for analysis. The incidence of CR in NSCLC patients treated with ICIs
was 1.5% (95% CI: 0.8–3.0) compared to 0.7% (95% CI: 0.4–1.2) in chemotherapy
(CT) groups. The use of ICIs in advanced NSCLC significantly improved the
possibility of archiving CR (RR 2.89, 95% CI: 1.44–5.81, P =0.003) compared
to CT. Subgroup analysis according to ICIs showed that the use of atezolizumab
(RR 3.26, P =0.01)
and nivolumab (RR 4.83, P =0.042) in advanced NSCLC significantly improved the
CR rate in comparison with CT alone, but not pembrolizumab and ipilimumab. We
also found that the use of ICIs as first-line (RR 2.39, 95% CI: 1.08–5.3, P =0.032) or
second-line (RR 4.99, 95% CI: 1.10–22.66, P =0.038) therapy
significantly increased the change in obtaining a CR. In addition, correlation
analysis indicates that PFS was strongly correlated with OS in NSCLC patients
who received ICIs (r =0.89 for PFS, P =0.017). No marked
correlation was found between OS and CR (r =0.19, P =0.75) and OS and ORR (r =0.52, P =0.28).
Conclusion: The CR is
a rate event in advanced NSCLC, but the use of ICIs significantly increases the
possibility of archiving CR in comparison with CT. PFS is significantly
correlated with OS and could be used as a surrogate end point, but not for CRs
and ORRs.
Keywords: immune
checkpoint inhibitors, non-small-cell lung cancer, complete response,
randomized controlled trials, meta-analysis, immunotherapy therapy, systematic
review