9 0 5 7 8
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 2.6 Breast Cancer (Dove Med Press)
- 3.9 Clin Epidemiol
- 3.3 Cancer Manag Res
- 3.9 Infect Drug Resist
- 3.6 Clin Interv Aging
- 4.8 Drug Des Dev Ther
- 2.8 Int J Chronic Obstr
- 8.0 Int J Nanomed
- 2.3 Int J Women's Health
- 3.2 Neuropsych Dis Treat
- 4.0 OncoTargets Ther
- 2.2 Patient Prefer Adher
- 2.8 Ther Clin Risk Manag
- 2.7 J Pain Res
- 3.3 Diabet Metab Synd Ob
- 4.3 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.9 Pharmgenomics Pers Med
- 3.5 Risk Manag Healthc Policy
- 4.5 J Inflamm Res
- 2.3 Int J Gen Med
- 4.1 J Hepatocell Carcinoma
- 3.2 J Asthma Allergy
- 2.3 Clin Cosmet Investig Dermatol
- 3.3 J Multidiscip Healthc

阿帕替尼联合多西紫杉醇治疗晚期食管癌的临床疗效及生存率分析
Authors Li J, Jia YX, Gao YP, Chang ZW, Han HQ, Yan J, Qin YR
Received 22 October 2018
Accepted for publication 29 January 2019
Published 8 April 2019 Volume 2019:12 Pages 2577—2583
DOI https://doi.org/10.2147/OTT.S191736
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Amy Norman
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev Srivastava
Background and aim: Standard
chemotherapy has limited clinical efficacy in patients with esophageal cancer
and there is a significant and unmet clinical need for effective treatment
options for these patients. The aim of this study was to compare the clinical
efficacy of the novel, targeted drug apatinib combined with docetaxel, and
docetaxel combined with S-1 as second- or further-line treatment for patients
with advanced esophageal cancer.
Methods: We
enrolled 33 patients with advanced esophageal cancer in chemotherapy group or
apatinib combined with chemotherapy group in this retrospective study. Apatinib
(500 mg) was taken orally once daily; docetaxel was administered at a dose of
75 mg/m2; and S-1 was optional at a dose of 40–60 mg, based on body surface
area. The primary endpoint of this study was progression-free survival (PFS).
Secondary endpoints included objective response rate (ORR), disease control
rate (DCR), and the incidence and severity of adverse events (AEs).
Results: No
complete response was observed in the two groups. However, two and five
patients achieved partial response in the chemotherapy group and the apatinib
combined with chemotherapy group, respectively. The ORR and DCR for the
chemotherapy group was 11.1% and 33.3%, respectively. In the apatinib
combination group, ORR and DCR was 88.9% and 93.3%, respectively. Anemia
(11.1%) and neutropenia (5.6%) were the most frequent grade III/IV AEs observed
in the chemotherapy group. In the apatinib combination group, the most frequent
grade III/IV AEs were anemia (13.3%), hypertension (6.7%), and proteinuria
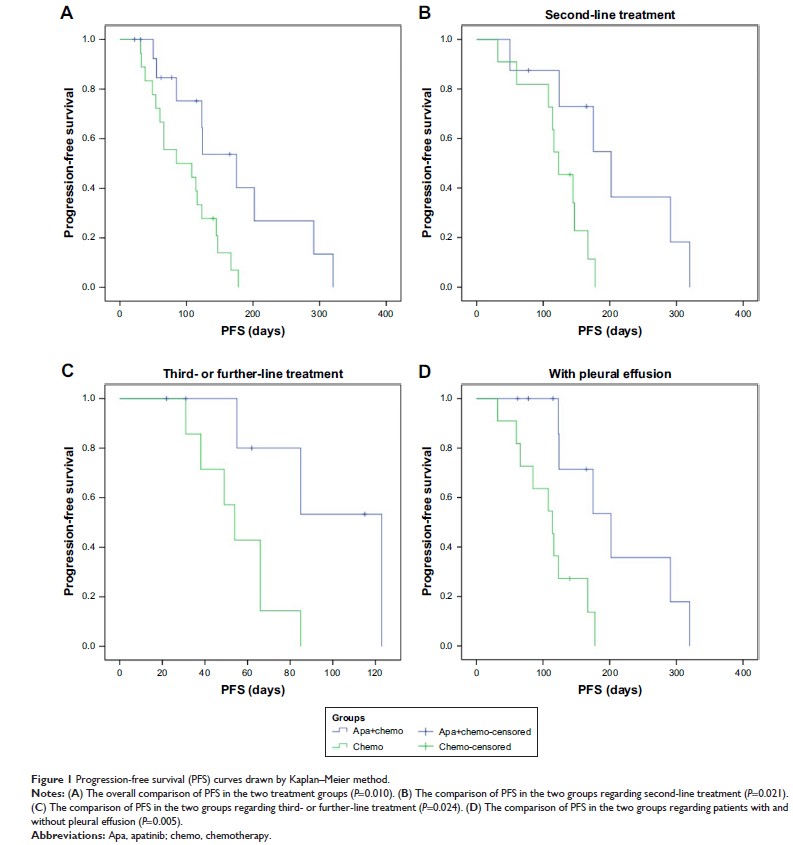
(6.7%). Median PFS was significantly longer in the apatinib combination group
than in the chemotherapy group (175 days vs 85 days, P =0.01).
Conclusion: The
combination of apatinib and docetaxel has a manageable toxicity profile and may
prolong survival. Therefore, this combination may be used as as second- or
further-line treatment for patients with advanced esophageal cancer.
Keywords: apatinib,
esophageal carcinoma, vascular endothelial growth factor, survival analysis