9 0 5 7 8
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 2.6 Breast Cancer (Dove Med Press)
- 3.9 Clin Epidemiol
- 3.3 Cancer Manag Res
- 3.9 Infect Drug Resist
- 3.6 Clin Interv Aging
- 4.8 Drug Des Dev Ther
- 2.8 Int J Chronic Obstr
- 8.0 Int J Nanomed
- 2.3 Int J Women's Health
- 3.2 Neuropsych Dis Treat
- 4.0 OncoTargets Ther
- 2.2 Patient Prefer Adher
- 2.8 Ther Clin Risk Manag
- 2.7 J Pain Res
- 3.3 Diabet Metab Synd Ob
- 4.3 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.9 Pharmgenomics Pers Med
- 3.5 Risk Manag Healthc Policy
- 4.5 J Inflamm Res
- 2.3 Int J Gen Med
- 4.1 J Hepatocell Carcinoma
- 3.2 J Asthma Allergy
- 2.3 Clin Cosmet Investig Dermatol
- 3.3 J Multidiscip Healthc

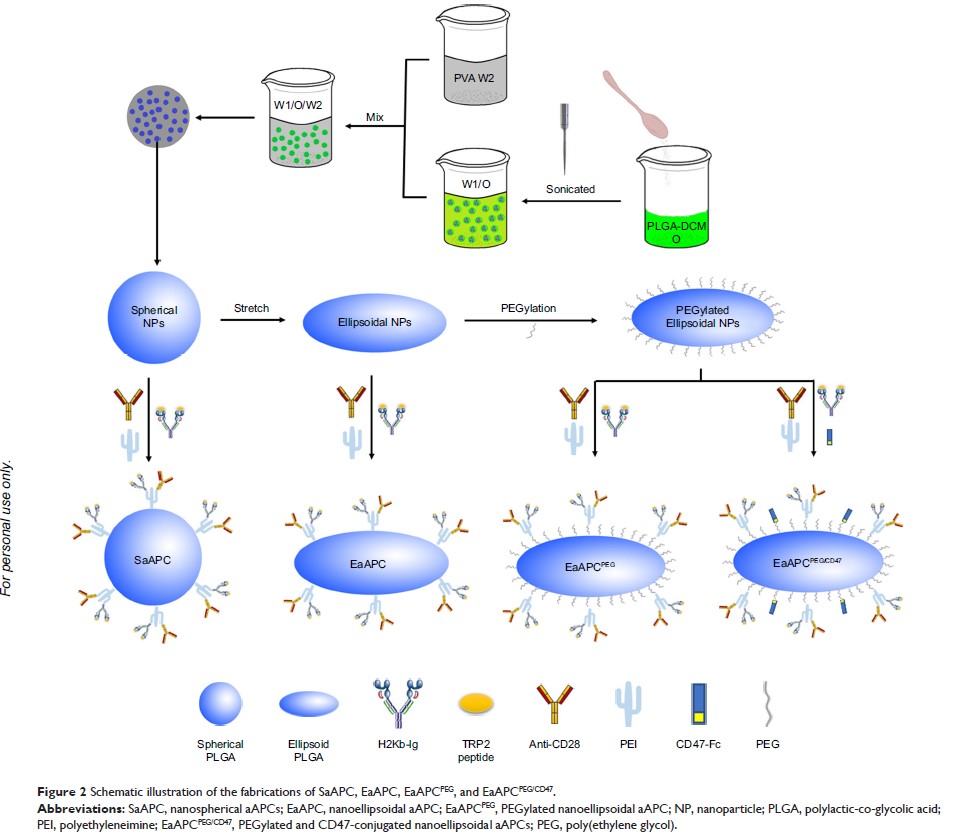
聚乙二醇化和 CD47 共轭的纳米脂质体人工抗原呈递细胞最大程度地减小吞噬作用并增强抗肿瘤T细胞反应
Authors Song S, Jin X, Zhang L, Zhao C, Ding Y, Ang Q, Khaidav O, Shen C
Received 24 November 2018
Accepted for publication 27 February 2019
Published 8 April 2019 Volume 2019:14 Pages 2465—2483
DOI https://doi.org/10.2147/IJN.S195828
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Cristina Weinberg
Peer reviewer comments 2
Editor who approved publication: Dr Mian Wang
Purpose: Antigen-presenting cells (APCs) are
powerful tools to expand antigen-specific T cells ex vivo and in vivo for tumor
immunotherapy, but suffer from time-consuming generation and biosafety concerns
raised by live cells. Alternatively, the cell-free artificial
antigen-presenting cells (aAPCs) have been rapidly developed. Nanoscale aAPCs
are recently proposed owing to their superior biodistribution and reduced
embolism than conventional cell-sized aAPCs, but pose the challenges: easier
cellular uptake and smaller contact surface area with T cells than the
cell-sized counterparts. This study aimed to fabricate a new “stealth”
nano-aAPCs with microscale contact surface area to minimize cellular uptake and
activate antigen-specific T cells by combination uses of ellipsoidal stretch,
PEGylation, and self-marker CD47-Fc conjugation.
Methods: The
spherical polylactic-co-glycolic acid nanoparticles were fabricated using a
double-emulsion method, and then stretched twofold using film-stretching
procedure followed by PEGylation and co-coupling with CD47-Fc, H-2Kb/TRP2180-188-Ig dimers,
and anti-CD28. The resulting PEGylated and CD47-conjugated nanoellipsoidal
aAPCs (EaAPCPEG/CD47) were co-cultured with macrophages or spleen
lymphocytes and also infused into melanoma-bearing mice. The in vitro and in
vivo effects were evaluated and compared with the nanospherical aAPCs (SaAPC),
nanoellipsoidal aAPCs (EaAPC), or PEGylated nanoellipsoidal aAPC (EaAPCPEG).
Results: EaAPCPEG/CD47 markedly reduced cellular uptake in
vitro and in vivo, as compared with EaAPCPEG, EaAPC, SaAPC,
and Blank-NPs and expanded naïve TRP2180-188-specific
CD8+ T cells in the co-cultures with spleen
lymphocytes. After three infusions, the EaAPCPEG/CD47 showed
much stronger effects on facilitating TRP2180-188-specific
CD8+ T-cell proliferation, local infiltration, and
tumor necrosis in the melanoma-bearing mice and on inhibiting tumor growth than
the control aAPCs.
Conclusion: The
superimposed or synergistic effects of ellipsoidal stretch, PEGylation, and
CD47-Fc conjugation minimized cellular uptake of nano-aAPCs and enhanced their
functionality to expand antigen-specific T cells and inhibit tumor growth, thus
suggesting a more valuable strategy to design “stealth” nanoscale aAPCs
suitable for tumor active immunotherapy.
Keywords: PLGA
nanoparticles, artificial antigen-presenting cells, phagocytosis, cancer active
immunotherapy