9 0 5 7 8
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 2.6 Breast Cancer (Dove Med Press)
- 3.9 Clin Epidemiol
- 3.3 Cancer Manag Res
- 3.9 Infect Drug Resist
- 3.6 Clin Interv Aging
- 4.8 Drug Des Dev Ther
- 2.8 Int J Chronic Obstr
- 8.0 Int J Nanomed
- 2.3 Int J Women's Health
- 3.2 Neuropsych Dis Treat
- 4.0 OncoTargets Ther
- 2.2 Patient Prefer Adher
- 2.8 Ther Clin Risk Manag
- 2.7 J Pain Res
- 3.3 Diabet Metab Synd Ob
- 4.3 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.9 Pharmgenomics Pers Med
- 3.5 Risk Manag Healthc Policy
- 4.5 J Inflamm Res
- 2.3 Int J Gen Med
- 4.1 J Hepatocell Carcinoma
- 3.2 J Asthma Allergy
- 2.3 Clin Cosmet Investig Dermatol
- 3.3 J Multidiscip Healthc

LPS 诱导的小窝蛋白-1 的磷酸化依赖性使跨细胞渗透性增加,其作用甚于细胞旁通透性的增加
Authors Wang N, Zhang D, Sun G, Zhang H, You Q, Shao M, Yue Y
Received 17 November 2014
Accepted for publication 7 January 2015
Published 28 August 2015 Volume 2015:9 Pages 4965—4977
DOI http://dx.doi.org/10.2147/DDDT.S77646
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 4
Editor who approved publication: Professor Wei Duan
Background: Lipopolysaccharide (LPS) was shown to induce an increase in caveolin-1 (Cav-1) expression in endothelial cells; however, the mechanisms regarding this response and the consequences on caveolae-mediated transcellular transport have not been completely investigated. This study aims to investigate the role of LPS-induced Cav-1 phosphorylation in pulmonary microvascular permeability in pulmonary microvascular endothelial cells (PMVECs).
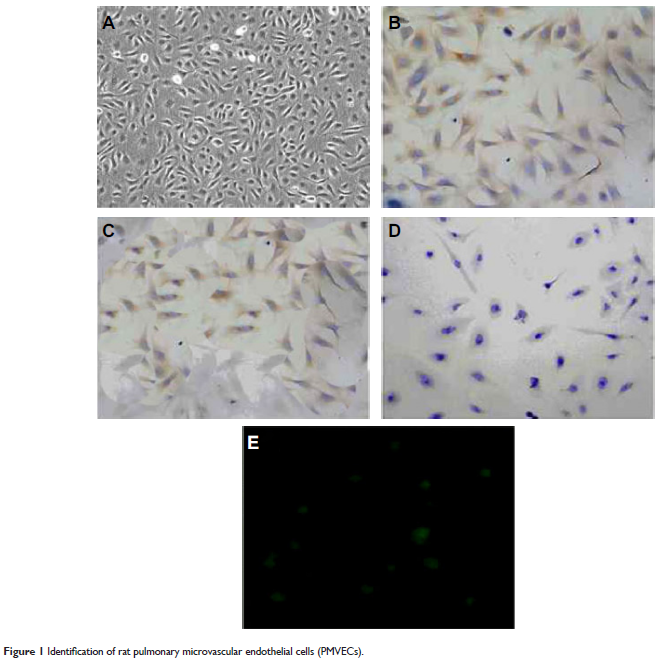
Methods: Rat PMVECs were isolated, cultured, and identified. Endocytosis experiments were employed to stain the nuclei by DAPI, and images were obtained with a fluorescence microscope. Permeability of endothelial cultures was measured to analyze the barrier function of endothelial monolayer. Western blot assay was used to examine the expression of Cav-1, pCav-1, triton-insoluble Cav-1, and triton-soluble Cav-1 protein.
Results: The LPS treatment induced phosphorylation of Cav-1, but did not alter the total Cav-1 level till 60 min in both rat and human PMVECs. LPS treatment also increased the triton-insoluble Cav-1 level, which peaked 15 min after LPS treatment in both rat and human PMVECs. LPS treatment increases the intercellular cell adhesion molecule-1 expression. Src inhibitors, including PP2, PP1, Saracatinib, and Quercetin, partially inhibited LPS-induced phosphorylation of Cav-1. In addition, both PP2 and caveolae disruptor MβCD inhibited LPS-induced increase of triton-insoluble Cav-1. LPS induces permeability by activating interleukin-8 and vascular endothelial growth factor and targeting other adhesion markers, such as ZO-1 and occludin. LPS treatment also significantly increased the endocytosis of albumin, which could be blocked by PP2 or MβCD. Furthermore, LPS treatment for 15 min significantly elevated Evans Blue-labeled BSA transport in advance of a decrease in transendothelial electrical resistance of PMVEC monolayer at this time point. After LPS treatment for 30 min, transendothelial electrical resistance decreased significantly. Moreover, PP2 and MβCD blocked LPS-induced increase in Evans Blue-labeled BSA level.
Conclusion: Our study demonstrates that LPS-induced Cav-1 phosphorylation may lead to the increase of transcellular permeability prior to the increase of paracellular permeability in a Src-dependent manner. Thus, LPS-induced Cav-1 phosphorylation may be a therapeutic target for the treatment of inflammatory lung disease associated with elevated microvascular permeability.
Keywords: caveolin-1, paracellular permeability, phosphorylation, pulmonary microvascular permeability, transcellular permeability