9 0 6 7 6
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 2.6 Breast Cancer (Dove Med Press)
- 3.9 Clin Epidemiol
- 3.3 Cancer Manag Res
- 3.9 Infect Drug Resist
- 3.6 Clin Interv Aging
- 4.8 Drug Des Dev Ther
- 2.8 Int J Chronic Obstr
- 8.0 Int J Nanomed
- 2.3 Int J Women's Health
- 3.2 Neuropsych Dis Treat
- 4.0 OncoTargets Ther
- 2.2 Patient Prefer Adher
- 2.8 Ther Clin Risk Manag
- 2.7 J Pain Res
- 3.3 Diabet Metab Synd Ob
- 4.3 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.9 Pharmgenomics Pers Med
- 3.5 Risk Manag Healthc Policy
- 4.5 J Inflamm Res
- 2.3 Int J Gen Med
- 4.1 J Hepatocell Carcinoma
- 3.2 J Asthma Allergy
- 2.3 Clin Cosmet Investig Dermatol
- 3.3 J Multidiscip Healthc

高糖会抑制 CIC-2 型氯通道,并减弱大鼠角质细胞迁移
Authors Pan F, Guo R, Cheng W, Chai L, Wang W, Cao C, Li S
Received 14 March 2015
Accepted for publication 1 May 2015
Published 28 August 2015 Volume 2015:9 Pages 4779—4791
DOI http://dx.doi.org/10.2147/DDDT.S84628
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 4
Editor who approved publication: Professor Wei Duan
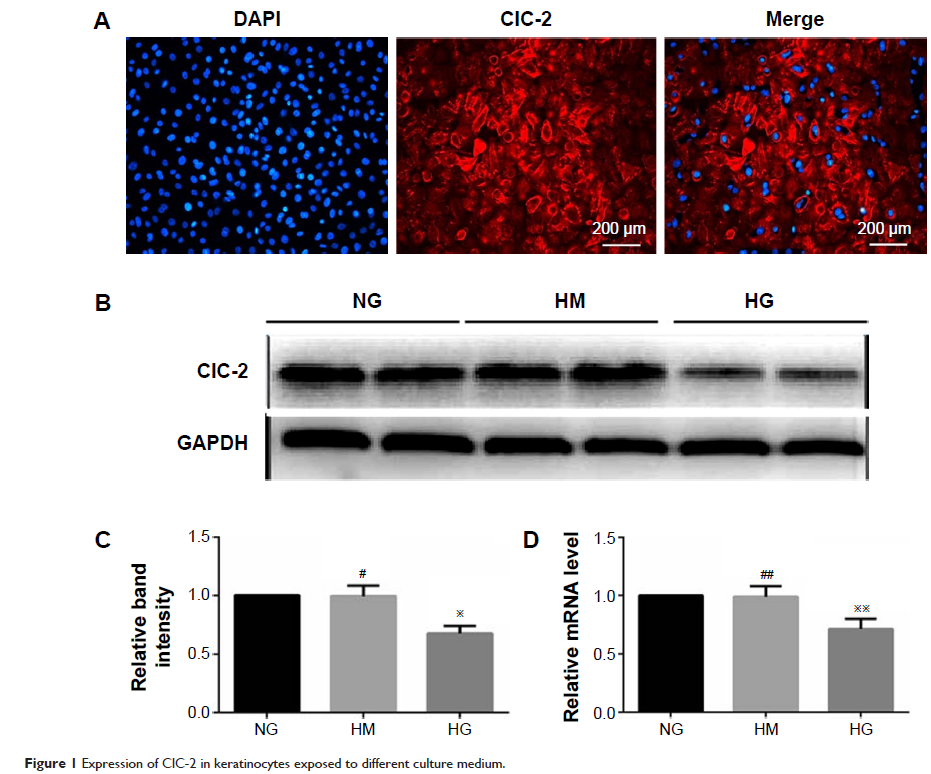
Background: Accumulating evidence has demonstrated that migration of keratinocytes is critical to wound epithelialization, and defects of this function result in chronic delayed-healing wounds in diabetes mellitus patients, and the migration has been proved to be associated with volume-activated chloride channels. The aim of the study is to investigate the effects of high glucose (HG, 25 mM) on ClC-2 chloride channels and cell migration of keratinocytes.
Methods: Newborn Sprague Dawley rats were used to isolate and culture the keratinocyte in this study. Immunofluorescence assay, real-time polymerase chain reaction, and Western blot assay were used to examine the expression of ClC-2 protein or mRNA. Scratch wound assay was used to measure the migratory ability of keratinocytes. Transwell cell migration assay was used to measure the invasion and migration of keratinocytes. Recombinant lentivirus vectors were established and transducted to keratinocytes. Whole-cell patch clamp was used to perform the electrophysiological studies.
Results: We found that the expression of ClC-2 was significantly inhibited when keratinocytes were exposed to a HG (25 mM) medium, accompanied by the decline of volume-activated Cl-current (I Cl,vol), migration potential, and phosphorylated PI3K as compared to control group. When knockdown of ClC-2 by RNAi or pretreatment with wortmannin, similar results were observed, including I Cl,vol and migration keratinocytes were inhibited.
Conclusion: Our study proved that HG inhibited ClC-2 chloride channels and attenuated cell migration of rat keratinocytes via inhibiting PI3K signaling.
Keywords: high glucose, keratinocytes, ClC-2, cell migration, PI3K