9 1 2 3 6
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 2.6 Breast Cancer (Dove Med Press)
- 3.9 Clin Epidemiol
- 3.3 Cancer Manag Res
- 3.9 Infect Drug Resist
- 3.6 Clin Interv Aging
- 4.8 Drug Des Dev Ther
- 2.8 Int J Chronic Obstr
- 8.0 Int J Nanomed
- 2.3 Int J Women's Health
- 3.2 Neuropsych Dis Treat
- 4.0 OncoTargets Ther
- 2.2 Patient Prefer Adher
- 2.8 Ther Clin Risk Manag
- 2.7 J Pain Res
- 3.3 Diabet Metab Synd Ob
- 4.3 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.9 Pharmgenomics Pers Med
- 3.5 Risk Manag Healthc Policy
- 4.5 J Inflamm Res
- 2.3 Int J Gen Med
- 4.1 J Hepatocell Carcinoma
- 3.2 J Asthma Allergy
- 2.3 Clin Cosmet Investig Dermatol
- 3.3 J Multidiscip Healthc

CRISPR-Cas9 系统可逆转由 mcr-1 -介导的粘菌素抗性,后者存在于大肠杆菌中
Authors Wan P, Cui S, Ma Z, Chen L, Li X, Zhao R, Xiong W, Zeng Z
Received 6 January 2020
Accepted for publication 30 March 2020
Published 22 April 2020 Volume 2020:13 Pages 1171—1178
DOI https://doi.org/10.2147/IDR.S244885
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 2
Editor who approved publication: Dr Joachim Wink
Purpose: The plasmid-borne mobilized colistin resistance gene (mcr-1 ) was discovered in 2015. Subsequently, the rapid horizontal transfer of mcr-1 gene to diverse bacterial species poses a serious threat to public health, which urgently needs the introduction of novel antimicrobial strategies. Therefore, the purpose of this study is to sensitize bacteria to colistin and reduce the propagation of mcr-1 gene by curing mcr-1 -harboring plasmid in Escherichia coli (E. coli ) using the CRISPR-Cas9 system.
Methods: Two sgRNAs specific to mcr-1 gene were designed and cloned into plasmid pCas9. The recombinant plasmid pCas9-mcr was transformed into E. coli carrying pUC19-mcr-1 or pHNSHP45, separately. The elimination efficiency in strains was evaluated by PCR and quantitative real-time PCR (qPCR). The antimicrobial susceptibility test was performed using the broth microdilution method.
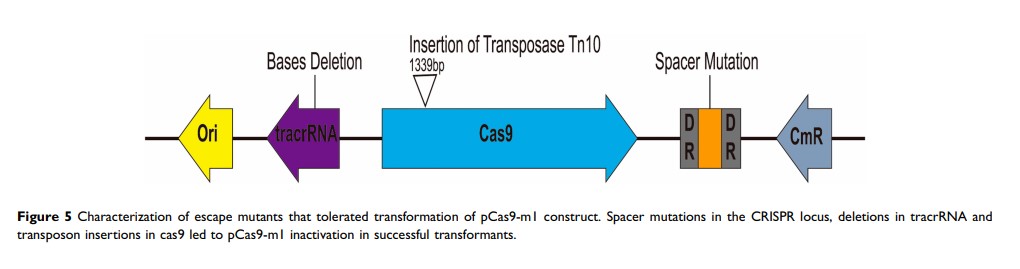
Results: In this study, we constructed the high copy number plasmid pUC19-mcr-1 and recombinant plasmid pCas9-m1 or pCas9-m2, which contain 20 nt or 30 nt sgRNA sequences targeted to mcr-1 , respectively. PCR and qPCR results showed that mcr-1 -harboring plasmids could be efficiently eliminated, and there was no significant correlation between sgRNA lengths and curing efficiency. However, when comparing restructured high copy number plasmid (pUC19-mcr-1 ) to natural resistance plasmid (pHNSHP45) in eliminating efficiency, we found that the content of plasmid backbone had an influence on efficiency. Furthermore, the conjugation assays verified that the engineered CRISPR-Cas9 system in bacteria or in bacteria genome can protect the recipient from plasmid-borne mcr-1 transfer via conjugation. Additionally, sequence analysis showed that three different types of defects in CRISPR-Cas9 system lead to escape mutants.
Conclusion: We presented a method that only one plasmid-mediated CRISPR-Cas9 system can be used to efficiently resensitize E. coli to colistin. Moreover, this system provided a great potentiality to counteract the propagation of mcr-1 among bacterial pathogens.
Keywords: sgRNA lengths, curing efficiency, backbone content, quantitative real-time PCR, conjugation assays, escape mutants