9 0 8 0 2
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 2.6 Breast Cancer (Dove Med Press)
- 3.9 Clin Epidemiol
- 3.3 Cancer Manag Res
- 3.9 Infect Drug Resist
- 3.6 Clin Interv Aging
- 4.8 Drug Des Dev Ther
- 2.8 Int J Chronic Obstr
- 8.0 Int J Nanomed
- 2.3 Int J Women's Health
- 3.2 Neuropsych Dis Treat
- 4.0 OncoTargets Ther
- 2.2 Patient Prefer Adher
- 2.8 Ther Clin Risk Manag
- 2.7 J Pain Res
- 3.3 Diabet Metab Synd Ob
- 4.3 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.9 Pharmgenomics Pers Med
- 3.5 Risk Manag Healthc Policy
- 4.5 J Inflamm Res
- 2.3 Int J Gen Med
- 4.1 J Hepatocell Carcinoma
- 3.2 J Asthma Allergy
- 2.3 Clin Cosmet Investig Dermatol
- 3.3 J Multidiscip Healthc

已发表论文
新型用于抗炎症制剂的吲哚 -2- 酮和 7- 氮杂 -2- 羟基吲哚衍生物的合成与生物评估
Authors Chen G, Jiang L, Dong L, Wang Z, Xu F, Ding T, Fu L, Fang Q, Liu Z, Shan X, Liang G
Published Date October 2014 Volume 2014:8 Pages 1869—1892
DOI http://dx.doi.org/10.2147/DDDT.S65997
Received 11 April 2014, Accepted 20 May 2014, Published 13 October 2014
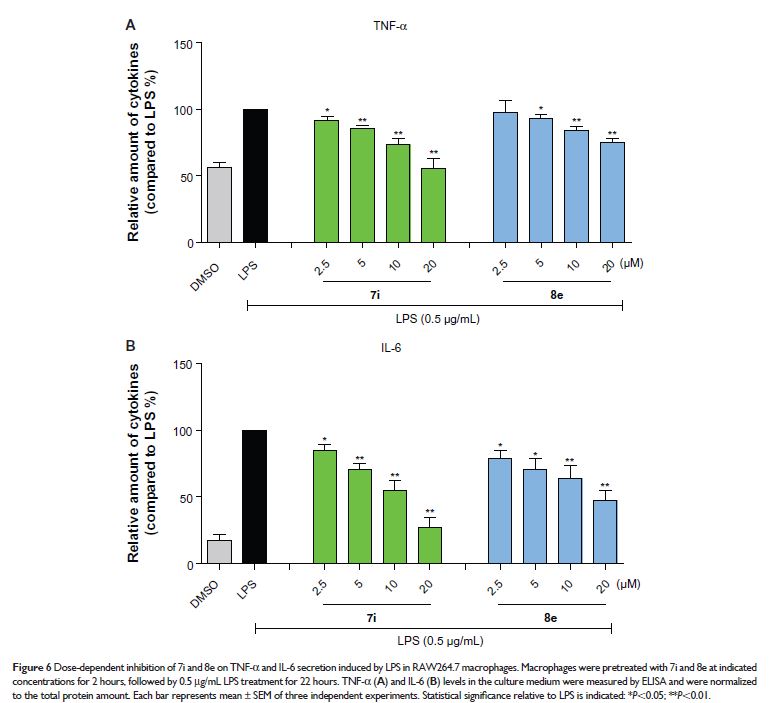
Abstract: Sepsis, a typically acute inflammatory disease, is the biggest cause of death in ICU (intensive care unit). Novel anti-inflammatory alternatives are still in urgent need. In this study, we designed and synthesized 30 indole-2-one and 7-aza-2-oxindole derivatives based on the skeleton of tenidap, and their anti-inflammatory activity was determined by evaluating the inhibitory potency against lipopolysaccharide (LPS)-stimulated tumor necrosis factor (TNF)-α and interleukin (IL)-6 release in RAW264.7 macrophages. Quantitative SAR (structure–activity relationship) analysis revealed that a high molecular polarizability and low lipid/water partition coefficient (ALogP) in indole-2-one are beneficial for anti-inflammatory activity. Moreover, compounds 7i and 8e inhibited the expression of TNF-α, IL-6, COX-2, PGES, and iNOS in LPS-stimulated macrophages, and 7i exhibited a significant protection from LPS-induced septic death in mouse models. These data present a series of new indole-2-one compounds with potential therapeutic effects in acute inflammatory diseases.
Keywords: anti-inflammation, macrophages, sepsis
Keywords: anti-inflammation, macrophages, sepsis