9 0 8 0 2
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 2.6 Breast Cancer (Dove Med Press)
- 3.9 Clin Epidemiol
- 3.3 Cancer Manag Res
- 3.9 Infect Drug Resist
- 3.6 Clin Interv Aging
- 4.8 Drug Des Dev Ther
- 2.8 Int J Chronic Obstr
- 8.0 Int J Nanomed
- 2.3 Int J Women's Health
- 3.2 Neuropsych Dis Treat
- 4.0 OncoTargets Ther
- 2.2 Patient Prefer Adher
- 2.8 Ther Clin Risk Manag
- 2.7 J Pain Res
- 3.3 Diabet Metab Synd Ob
- 4.3 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.9 Pharmgenomics Pers Med
- 3.5 Risk Manag Healthc Policy
- 4.5 J Inflamm Res
- 2.3 Int J Gen Med
- 4.1 J Hepatocell Carcinoma
- 3.2 J Asthma Allergy
- 2.3 Clin Cosmet Investig Dermatol
- 3.3 J Multidiscip Healthc

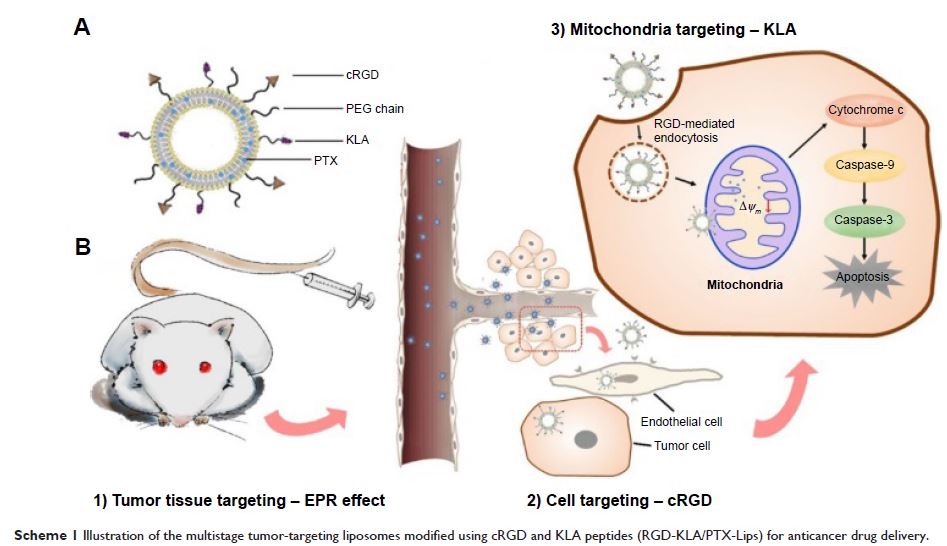
使用经 RGD 和 KLA 肽修饰的多阶段肿瘤靶向脂质体提高紫杉醇 (paclitaxel) 的抗癌功效
Authors Sun J, Jiang L, Lin Y, Gerhard EM, Jiang X, Li L, Yang J, Gu Z
Received 21 September 2016
Accepted for publication 4 January 2017
Published 27 February 2017 Volume 2017:12 Pages 1517—1537
DOI https://doi.org/10.2147/IJN.S122859
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Alexander Kharlamov
Peer reviewer comments 4
Editor who approved publication: Dr Linlin Sun
Abstract: Mitochondria serve as both “energy factories” and “suicide weapon
stores” of cells. Targeted delivery of cytotoxic drugs to the mitochondria of
tumor cells and tumor vascular cells is a promising strategy to improve the
efficacy of chemotherapy. Here, multistage tumor-targeting liposomes containing
two targeted peptide-modified lipids, cRGD-PEG2000-DSPE and KLA-PEG2000-DSPE,
were developed for encapsulation of the anticancer drug paclitaxel (PTX,
RGD-KLA/PTX-Lips). Compared with Taxol (free PTX), RGD/PTX-Lips and
KLA/PTX-Lips, the half-maximal inhibitory concentration (IC50) value of RGD-KLA/PTX-Lips in vitro was 1.9-,
36.7- and 22.7-fold lower with 4T1 cells, respectively, because of higher
levels of cellular uptake. Similar results were also observed with human
umbilical vascular endothelial cells (HUVECs). An apoptosis assay showed that
the total apoptotic ratio of RGD-KLA/PTX-Lips was the highest because of the
mitochondria-targeted drug delivery and the activation of mitochondrial
apoptosis pathways, as evidenced by visible mitochondrial localization,
decreased mitochondrial membrane potential, release of cytochrome c and
increased activities of caspase-9 and caspase-3. The strongest tumor growth
inhibition (TGI; 80.6%) and antiangiogenesis effects without systemic toxicity
were also observed in RGD-KLA/PTX-Lip-treated 4T1 tumor xenograft BALB/c mice.
In conclusion, these multistage tumor-targeting liposomes represent a promising
anticancer drug delivery system (DDS) capable of maximizing anticancer
therapeutic efficacy and minimizing systemic toxicity.
Keywords: multistage tumor-targeting liposome,
mitochondria, paclitaxel, anticancer, antiangiogenesis