9 0 8 0 2
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 2.6 Breast Cancer (Dove Med Press)
- 3.9 Clin Epidemiol
- 3.3 Cancer Manag Res
- 3.9 Infect Drug Resist
- 3.6 Clin Interv Aging
- 4.8 Drug Des Dev Ther
- 2.8 Int J Chronic Obstr
- 8.0 Int J Nanomed
- 2.3 Int J Women's Health
- 3.2 Neuropsych Dis Treat
- 4.0 OncoTargets Ther
- 2.2 Patient Prefer Adher
- 2.8 Ther Clin Risk Manag
- 2.7 J Pain Res
- 3.3 Diabet Metab Synd Ob
- 4.3 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.9 Pharmgenomics Pers Med
- 3.5 Risk Manag Healthc Policy
- 4.5 J Inflamm Res
- 2.3 Int J Gen Med
- 4.1 J Hepatocell Carcinoma
- 3.2 J Asthma Allergy
- 2.3 Clin Cosmet Investig Dermatol
- 3.3 J Multidiscip Healthc

作为体外和体内有效抗菌剂的自组装纳米杆菌肽的合成、构建和评价
Authors Hong W, Gao X, Qiu P, Yang J, Qiao M, Shi H, Zhang D, Tian C, Niu S, Liu M
Received 15 March 2017
Accepted for publication 7 June 2017
Published 30 June 2017 Volume 2017:12 Pages 4691—4708
DOI https://doi.org/10.2147/IJN.S136998
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Alexander Kharlamov
Peer reviewer comments 3
Editor who approved publication: Dr Linlin Sun
Abstract: Bacitracin A (BA) is an excellent
polypeptide antibiotic that is active against gram-positive bacteria without
triggering multidrug resistance. However, BA is inactive against gram-negative
bacteria because of its inability to cross the outer membrane of these cells,
and it has strong nephrotoxicity, thus limiting its clinical applications.
Nanoantibiotics can effectively localize antibiotics to the periplasmic space
of bacteria while decreasing the adverse effects of antibiotics. In this study,
biodegradable hydrophobic copolymers of poly (D,L-lactide-co-glycolide) (PLGA)
were attached to the N-termini of BA to design a novel class of self-assembled
nano-bacitracin A (nano-BAs), and their potential as antibacterial agents was
evaluated in vitro and in vivo. Nano-BAs had a core-shell structure with a mean
diameter <150 nm. Impressively, nano-BAs had strong antibacterial properties
against both gram-positive and gram-negative bacteria, and the distribution of
antibacterial activity as a function of PLGA block length was skewed toward
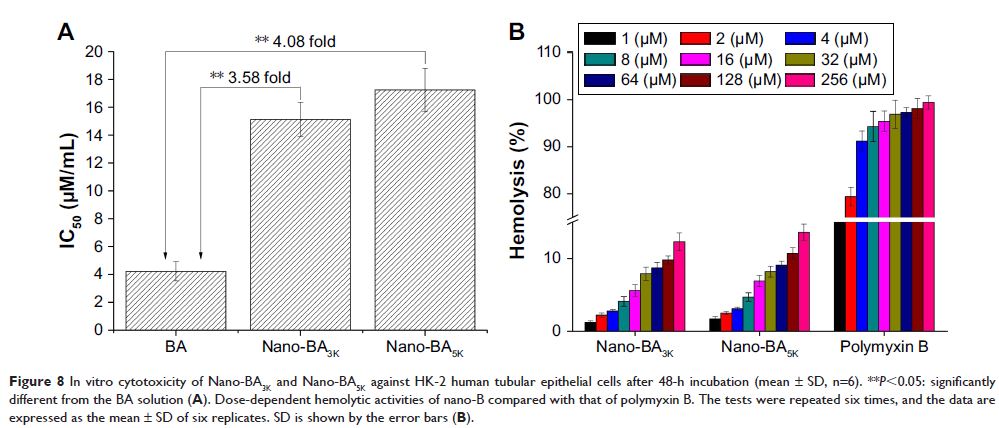
longer PLGA chains. No cytotoxicity against HK-2 cells or human red blood cells
(hRBCs) was observed in vitro, suggesting good biocompatibility. A high local
density of BA mass on the surface promoted endocytotic cellular uptake, and
hydrophobic interactions between the PLGA block and lipopolysaccharide (LPS)
facilitated the uptake of nano-BAs, thereby leading to greater antibacterial
activities. In addition, Nano-BA5K was found
to be effective in vivo, and it served as an anti-infective agent for wound healing.
Collectively, this study provides a cost-effective means of developing
self-assembling nano-polypeptide antibiotic candidates with a broader
antibacterial spectrum and a lower toxicity than commercially available peptide
antibiotics, owing to their modification with biodegradable copolymers.
Keywords: bacitracin A,
poly (D,L-lactide-co-glycolide), nano-BAs, broader antibacterial spectrum,
wound healing