9 0 8 0 2
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 2.6 Breast Cancer (Dove Med Press)
- 3.9 Clin Epidemiol
- 3.3 Cancer Manag Res
- 3.9 Infect Drug Resist
- 3.6 Clin Interv Aging
- 4.8 Drug Des Dev Ther
- 2.8 Int J Chronic Obstr
- 8.0 Int J Nanomed
- 2.3 Int J Women's Health
- 3.2 Neuropsych Dis Treat
- 4.0 OncoTargets Ther
- 2.2 Patient Prefer Adher
- 2.8 Ther Clin Risk Manag
- 2.7 J Pain Res
- 3.3 Diabet Metab Synd Ob
- 4.3 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.9 Pharmgenomics Pers Med
- 3.5 Risk Manag Healthc Policy
- 4.5 J Inflamm Res
- 2.3 Int J Gen Med
- 4.1 J Hepatocell Carcinoma
- 3.2 J Asthma Allergy
- 2.3 Clin Cosmet Investig Dermatol
- 3.3 J Multidiscip Healthc

调强放射治疗加尼妥珠单抗联合或不联合化疗治疗局部晚期鼻咽癌
Authors Huang JF, Zou QZ, Qian DQ, Zhou LY, Yang B, Chu JJ, Pang QF, Wang KW, Zhang FZ
Received 13 September 2017
Accepted for publication 6 November 2017
Published 8 December 2017 Volume 2017:10 Pages 5835—5841
DOI https://doi.org/10.2147/OTT.S151554
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Akshita Wason
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Geoffrey Pietersz
Objective: This study aimed to evaluate the safety and efficacy of
intensity-modulated radiotherapy (IMRT) plus nimotuzumab with or without
concurrent chemotherapy (CCT) for patients with locally advanced nasopharyngeal
carcinoma (LA-NPC).
Patients and methods: A total of 50 newly diagnosed patients with
LA-NPC treated at the Affiliated Hospital of Jiangnan University between
November 2011 and January 2017 were retrospectively analyzed. All patients
received the combined treatment modality of nimotuzumab plus IMRT. Nimotuzumab
was administered concurrently with IMRT at a weekly dose of 200 mg.
Neoadjuvant, concurrent or adjuvant chemotherapy with the doublet regimen of
taxanes (docetaxel or paclitaxel) plus platinum (cisplatin or nedaplatin) were
administered. Among the 50 patients, 43 (86.0%) received ≥6 cycles of
nimotuzumab (median 7 cycles, range 2–14 cycles) and 29 (58.0%) received two
cycles of CCT with docetaxel plus nedaplatin.
Results: With a median follow-up of 28.0 months, the 2-year
progression-free survival (PFS) and overall survival were 83.29% (95%
confidence interval [CI]: 67.93%–91.72%) and 97.67% (95% CI: 84.62%–99.67%),
respectively. Both univariate and multivariate analyses revealed that cycles of
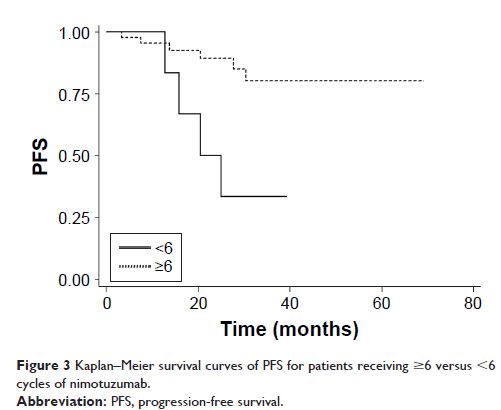
nimotuzumab were significantly associated with PFS. Patients who
received ≥6 cycles of nimotuzumab showed a better PFS than those
receiving <6 cycles (P =0.006), whereas
the addition of CCT failed to improve PFS. Oral mucositis was the most common
adverse event, which was recorded as grade 3–4 in 18 (36.0%) patients. Besides,
two (4.0%) patients experienced nimotuzumab-related anaphylaxis, and no skin
rash was found in any patient. Subgroup analysis revealed that the patients who
received CCT had more grade 3–4 adverse events as compared to those who did not
receive CCT (62.1% vs 33.3%, P =0.045).
Conclusion: The regime of nimotuzumab plus IMRT for the
treatment of LA-NPC was well tolerated, with encouraging survival data, and it
could be an effective treatment alternative for patients with LA-NPC. Further
clinical trials are needed to confirm these findings.
Keywords: nasopharyngeal
carcinoma, intensity-modulated radiotherapy, nimotuzumab, concurrent
chemoradiotherapy, treatment outcome