9 0 8 0 2
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 2.6 Breast Cancer (Dove Med Press)
- 3.9 Clin Epidemiol
- 3.3 Cancer Manag Res
- 3.9 Infect Drug Resist
- 3.6 Clin Interv Aging
- 4.8 Drug Des Dev Ther
- 2.8 Int J Chronic Obstr
- 8.0 Int J Nanomed
- 2.3 Int J Women's Health
- 3.2 Neuropsych Dis Treat
- 4.0 OncoTargets Ther
- 2.2 Patient Prefer Adher
- 2.8 Ther Clin Risk Manag
- 2.7 J Pain Res
- 3.3 Diabet Metab Synd Ob
- 4.3 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.9 Pharmgenomics Pers Med
- 3.5 Risk Manag Healthc Policy
- 4.5 J Inflamm Res
- 2.3 Int J Gen Med
- 4.1 J Hepatocell Carcinoma
- 3.2 J Asthma Allergy
- 2.3 Clin Cosmet Investig Dermatol
- 3.3 J Multidiscip Healthc

含阿司匹林壳聚糖纳米粒子的不对称胶原 - 壳聚糖膜引导骨再生
Authors Zhang J, Ma S, Liu Z, Geng H, Lu X, Zhang X, Li H, Gao C, Zhang X, Gao P
Received 3 August 2017
Accepted for publication 10 November 2017
Published 15 December 2017 Volume 2017:12 Pages 8855—8866
DOI https://doi.org/10.2147/IJN.S148179
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Alexander Kharlamov
Peer reviewer comments 2
Editor who approved publication: Dr Linlin Sun
Introduction: Membranes allowing the sustained release of drugs that can achieve
cell adhesion are very promising for guided bone regeneration. Previous studies
have suggested that aspirin has the potential to promote bone regeneration. The
purpose of this study was to prepare a local drug delivery system with
aspirin-loaded chitosan nanoparticles (ACS) contained in an asymmetric
collagen-chitosan membrane (CCM).
Methods: In this study, the ACS were fabricated using
different concentrations of aspirin (5 mg, 25 mg, 50 mg, and 75 mg). The drug
release behavior of ACS was studied. Transmission electron microscopy (TEM) and
scanning electron microscopy (SEM) were used to examine the micromorphology of
ACS and aspirin-loaded chitosan nanoparticles contained in chitosan-collagen
membranes (ACS-CCM). In vitro bone mesenchymal stem cells (BMSCs) were cultured
and critical-sized cranial defects on Sprague-Dawley rats were made to evaluate
the effect of the ACS-CCM on bone regeneration.
Results: Drug release behavior results of ACS showed that
the nanoparticles fabricated in this study could successfully sustain the
release of the drug. TEM showed the morphology of the nanoparticles. SEM images
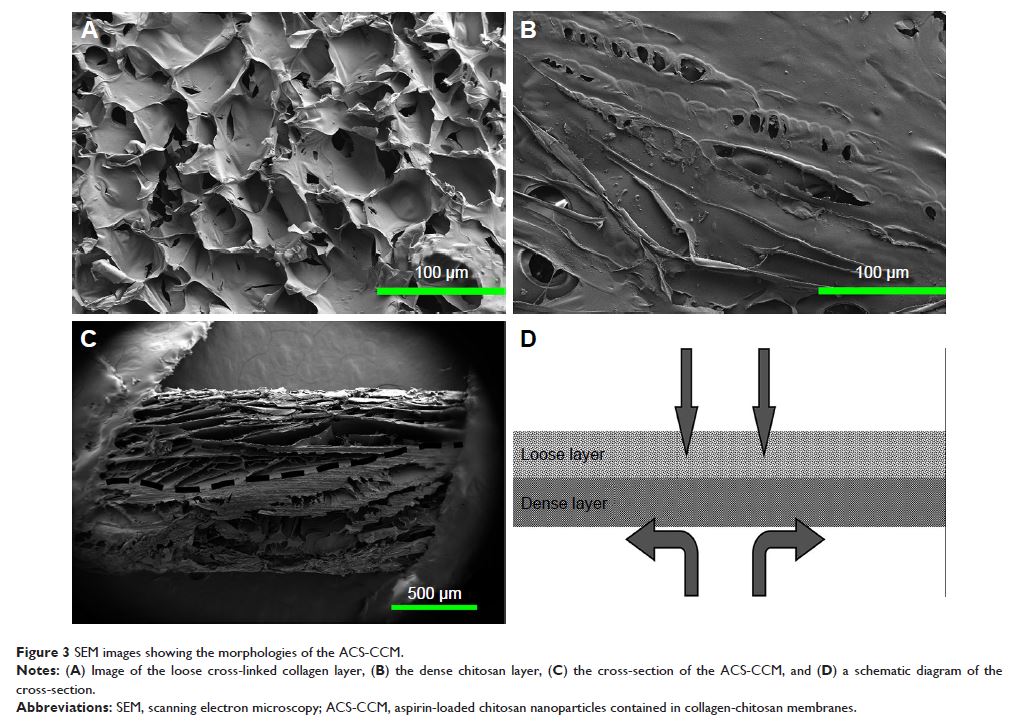
indicated that the asymmetric membrane comprised a loose collagen layer and a
dense chitosan layer. In vitro studies showed that ACS-CCM could promote the
proliferation of BMSCs, and that the degree of differentiated BMSCs seeded on
CCMs containing 50 mg of ACS was higher than that of other membranes.
Micro-computed tomography showed that 50 mg of ACS-CCM resulted in enhanced
bone regeneration compared with the control group.
Conclusion: This study shows that the ACS-CCM would allow
the sustained release of aspirin and have further osteogenic potential. This
membrane is a promising therapeutic approach to guiding bone regeneration.
Keywords: aspirin,
nanoparticle, drug release, membrane, guided bone regeneration