9 0 8 0 2
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 2.6 Breast Cancer (Dove Med Press)
- 3.9 Clin Epidemiol
- 3.3 Cancer Manag Res
- 3.9 Infect Drug Resist
- 3.6 Clin Interv Aging
- 4.8 Drug Des Dev Ther
- 2.8 Int J Chronic Obstr
- 8.0 Int J Nanomed
- 2.3 Int J Women's Health
- 3.2 Neuropsych Dis Treat
- 4.0 OncoTargets Ther
- 2.2 Patient Prefer Adher
- 2.8 Ther Clin Risk Manag
- 2.7 J Pain Res
- 3.3 Diabet Metab Synd Ob
- 4.3 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.9 Pharmgenomics Pers Med
- 3.5 Risk Manag Healthc Policy
- 4.5 J Inflamm Res
- 2.3 Int J Gen Med
- 4.1 J Hepatocell Carcinoma
- 3.2 J Asthma Allergy
- 2.3 Clin Cosmet Investig Dermatol
- 3.3 J Multidiscip Healthc

冷冻治疗通过恢复肿瘤引流淋巴结中由肿瘤引发的树突状细胞来提高抗肿瘤免疫力
Authors He XZ, Wang QF, Han S, Wang HQ, Ye YY, Zhu ZY, Zhang SZ
Published Date March 2015 Volume 2015:9 Pages 1449—1458
DOI http://dx.doi.org/10.2147/DDDT.S76592
Received 29 October 2014, Accepted 21 November 2014, Published 10 March 2015
Approved for publication by Dr Wei Duan
Background: In addition to minimally invasive destruction of tumors, cryo-ablation of tumors to some extent modulated anti-tumor immunity. Cryo-ablated tumors in glioma mice models induced anti-tumor cellular immunologic response which increases the percentage of CD3+ and CD4+T cells in blood as well as natural killer cells. As a crucial role in triggering anti-tumor immunity, dendritic cells (DCs) were educated by tumors to adopt a tolerance phenotype which helps the tumor escape from immune monitoring. This study aims to study whether cryo-ablation could influence the tolerogenic DCs, and influence anti-tumor immunity in tumor-draining lymph nodes (TDLNs).
Methods: Using the GL261 subcutaneous glioma mouse model, we created a tumor bearing group, cryo-ablation group, and surgery group. We analyzed alteration in phenotype and function of tolerogenic DCs, and evaluated the factors of anti-tumor immunity inhibition.
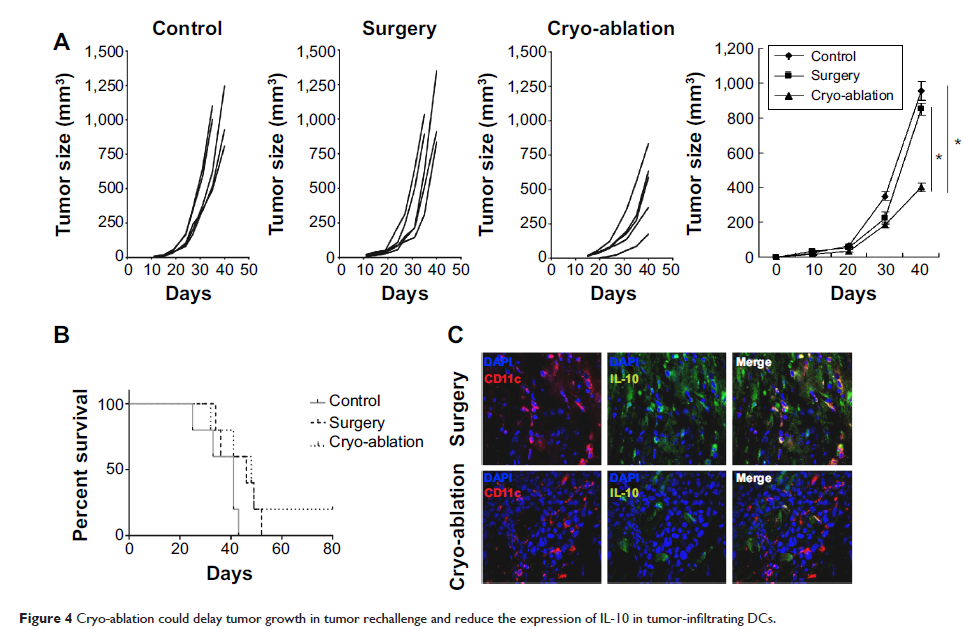
Results: DCs in TDLNs in GL261 subcutaneous glioma mouse model expressed tolerogenic phenotype. In contrast to surgery, cryo-ablation improved the quantity and quality of these tolerogenic DCs. Moreover, the DCs decreased the expression of intracellular interleukin-10 (IL-10) and extra-cellular IL-10. In vitro, DCs from the cryo-ablation group recovered their specific function and induced potent anti-tumor immunity through triggering T cells. In vivo, cryo-ablation showed weak anti-tumor immunity, only inhibiting the growth of rechallenged tumors. But many IL-10-low DCs, rather than IL-10-high DCs, infiltrated the tumors. More importantly, Tregs inhibited the performance of these DCs; and depletion of Tregs greatly improved anti-tumor immunity in vivo.
Conclusion: Cryo-ablation could recover function of tumor induced tolerogenic DCs in vitro; and depletion of Tregs could improve this anti-tumor effect in vivo. The Tregs/CD4+T and Tregs/CD25+T cells in TDLNs inhibit DCs’ activity and function.
Keywords: glioma, cryo-ablation, dendritic cells, tumor-draining lymph nodes, anti-tumor immunity