9 1 2 3 6
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 2.6 Breast Cancer (Dove Med Press)
- 3.9 Clin Epidemiol
- 3.3 Cancer Manag Res
- 3.9 Infect Drug Resist
- 3.6 Clin Interv Aging
- 4.8 Drug Des Dev Ther
- 2.8 Int J Chronic Obstr
- 8.0 Int J Nanomed
- 2.3 Int J Women's Health
- 3.2 Neuropsych Dis Treat
- 4.0 OncoTargets Ther
- 2.2 Patient Prefer Adher
- 2.8 Ther Clin Risk Manag
- 2.7 J Pain Res
- 3.3 Diabet Metab Synd Ob
- 4.3 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.9 Pharmgenomics Pers Med
- 3.5 Risk Manag Healthc Policy
- 4.5 J Inflamm Res
- 2.3 Int J Gen Med
- 4.1 J Hepatocell Carcinoma
- 3.2 J Asthma Allergy
- 2.3 Clin Cosmet Investig Dermatol
- 3.3 J Multidiscip Healthc

已发表论文
在慢加急性肝衰竭患者治疗中,体外细胞疗法 (ELAD®) 与随机化控制临床试验的标准治疗的比较
Authors Duan Z, Xin S, Zhang J, You S, Chen Y, Liu H, Zheng S, Li Z, Ashley R, Millis M
Received 14 July 2018
Accepted for publication 3 October 2018
Published 16 November 2018 Volume 2018:10 Pages 139—152
DOI https://doi.org/10.2147/HMER.S180246
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Amy Norman
Peer reviewer comments 3
Editor who approved publication: Dr Gerry Lake-Bakaar
Background: Preliminary evidence of safety and
efficacy of an extracorporeal cellular therapy (ELAD®) has been demonstrated in
subjects with acute forms of liver failure. This study compared ELAD with
standard of care in Chinese subjects with acute-on-chronic liver failure
(ACLF), predominantly secondary to chronic viral hepatitis.
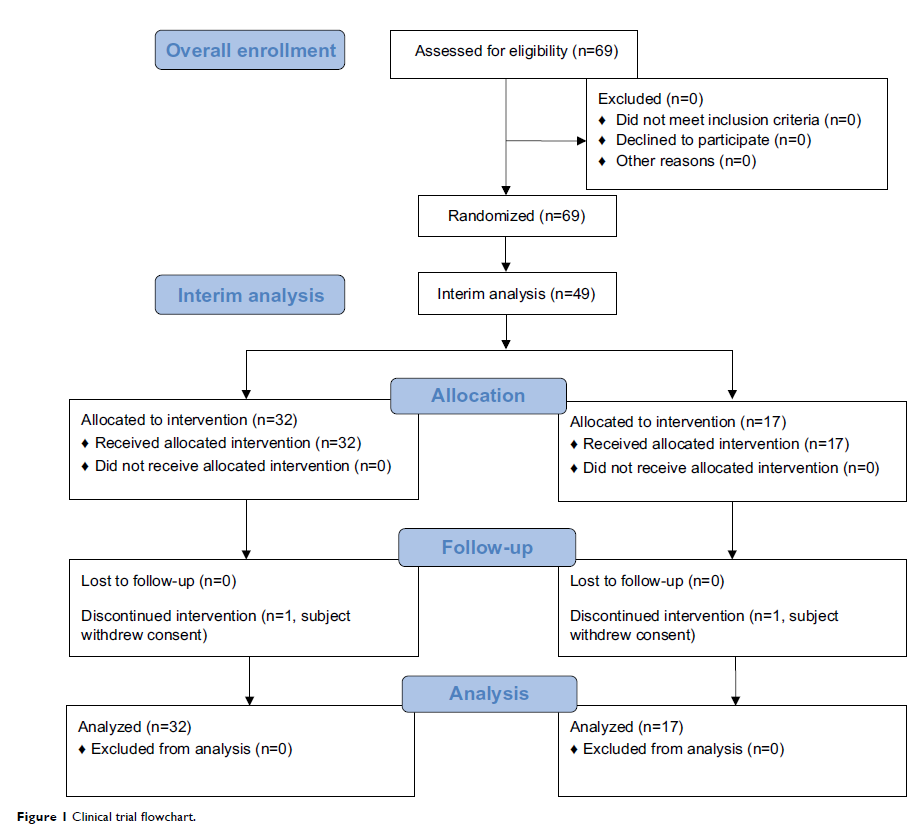
Subjects and methods: Subjects meeting eligibility criteria were randomized to either the ELAD group or the control group. All subjects received plasma exchange and venovenous hemofiltration and either ELAD treatment for 3–5 days, unless terminated early, along with standard of care or standard of care alone (control) and were then followed up for 12 weeks.
Results: Forty-nine subjects (ELAD subjects, 32; controls, 17) were randomized under this protocol. Kaplan–Meier analysis of transplant-free survival (TFS) revealed a significant difference in favor of ELAD vs control (P =0.049, Wilcoxon signed-rank test). There was a significant difference in TFS on day 28 in ELAD vs control (P =0.022). In a multiple regression model, the relationship between group assignment and outcome was significant (P =0.031) when changes in food intake and Model for End-Stage Liver Disease (MELD) scores at screening were included as additional independent variables. The duration of ELAD treatment alone was a significant predictor of TFS (P =0.043). Median time to a 5-point increase in MELD, transplant, or death was longer than 72 days with ELAD vs 26 days for control (P =0.036). Total bilirubin level decreased by 25% during ELAD treatment vs 37% increase in the control group (P <0.001) over an equivalent period. Adverse events attributed to the ELAD system were expected and could be managed conservatively. Intergroup differences in certain vital signs and laboratory parameters were noted during treatment and generally resolved posttreatment.
Conclusion: ELAD treatment was well tolerated by Chinese subjects with ACLF, predominately secondary to chronic viral hepatitis. Results demonstrate a significant improvement in TFS in ELAD vs control groups in association with significant improvements in serum bilirubin levels presumably related to improvement in hepatic function.
Keywords: acute-on-chronic liver failure, ELAD, bioartificial liver support, ACLF, C3A cells, cellular therapy
Subjects and methods: Subjects meeting eligibility criteria were randomized to either the ELAD group or the control group. All subjects received plasma exchange and venovenous hemofiltration and either ELAD treatment for 3–5 days, unless terminated early, along with standard of care or standard of care alone (control) and were then followed up for 12 weeks.
Results: Forty-nine subjects (ELAD subjects, 32; controls, 17) were randomized under this protocol. Kaplan–Meier analysis of transplant-free survival (TFS) revealed a significant difference in favor of ELAD vs control (P =0.049, Wilcoxon signed-rank test). There was a significant difference in TFS on day 28 in ELAD vs control (P =0.022). In a multiple regression model, the relationship between group assignment and outcome was significant (P =0.031) when changes in food intake and Model for End-Stage Liver Disease (MELD) scores at screening were included as additional independent variables. The duration of ELAD treatment alone was a significant predictor of TFS (P =0.043). Median time to a 5-point increase in MELD, transplant, or death was longer than 72 days with ELAD vs 26 days for control (P =0.036). Total bilirubin level decreased by 25% during ELAD treatment vs 37% increase in the control group (P <0.001) over an equivalent period. Adverse events attributed to the ELAD system were expected and could be managed conservatively. Intergroup differences in certain vital signs and laboratory parameters were noted during treatment and generally resolved posttreatment.
Conclusion: ELAD treatment was well tolerated by Chinese subjects with ACLF, predominately secondary to chronic viral hepatitis. Results demonstrate a significant improvement in TFS in ELAD vs control groups in association with significant improvements in serum bilirubin levels presumably related to improvement in hepatic function.
Keywords: acute-on-chronic liver failure, ELAD, bioartificial liver support, ACLF, C3A cells, cellular therapy