9 0 8 1 0
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 2.6 Breast Cancer (Dove Med Press)
- 3.9 Clin Epidemiol
- 3.3 Cancer Manag Res
- 3.9 Infect Drug Resist
- 3.6 Clin Interv Aging
- 4.8 Drug Des Dev Ther
- 2.8 Int J Chronic Obstr
- 8.0 Int J Nanomed
- 2.3 Int J Women's Health
- 3.2 Neuropsych Dis Treat
- 4.0 OncoTargets Ther
- 2.2 Patient Prefer Adher
- 2.8 Ther Clin Risk Manag
- 2.7 J Pain Res
- 3.3 Diabet Metab Synd Ob
- 4.3 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.9 Pharmgenomics Pers Med
- 3.5 Risk Manag Healthc Policy
- 4.5 J Inflamm Res
- 2.3 Int J Gen Med
- 4.1 J Hepatocell Carcinoma
- 3.2 J Asthma Allergy
- 2.3 Clin Cosmet Investig Dermatol
- 3.3 J Multidiscip Healthc

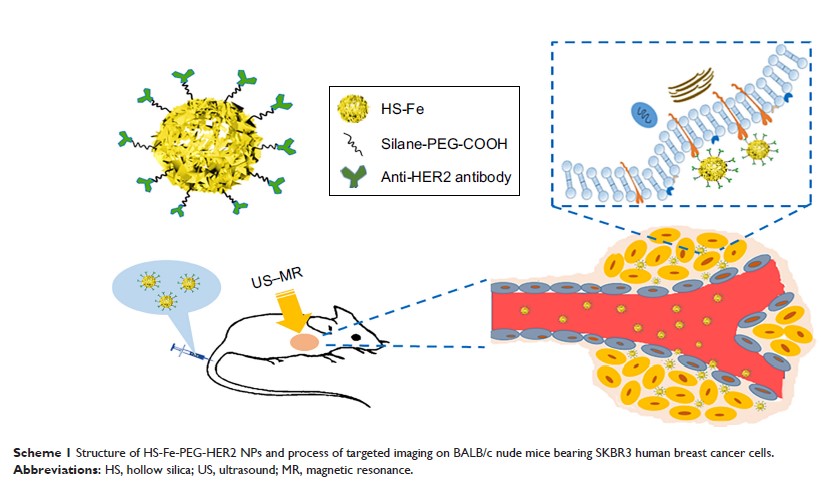
靶向铁掺杂二氧化硅纳米粒子作为 HER2 阳性乳腺癌的新型超声 - 磁共振双模成像造影剂
Authors Li X, Xia S, Zhou W, Ji R, Zhan W
Received 30 September 2018
Accepted for publication 3 February 2019
Published 5 April 2019 Volume 2019:14 Pages 2397—2413
DOI https://doi.org/10.2147/IJN.S189252
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Govarthanan Muthusamy
Peer reviewer comments 5
Editor who approved publication: Dr Lei Yang
Background: Multimodal
contrast agents with low toxicity and targeted modification have opened up new
possibilities for specific imaging of breast cancer and shown broad application
prospects in biomedicine and great potential for clinical transformation. In
this work, a potential multifunctional imaging agent was developed by doping Fe
into hollow silica nanoparticles (HS-Fe NPs), followed by modification with
specific anti-HER2 antibodies, enabling the NPs to have dual-mode ultrasound
(US)–magnetic resonance (MR)-specific imaging capacity with low toxicity.
Methods: Anti-HER2
antibodies were conjugated to silane–polyethylene glycol (PEG)–COOH-modified
HS-Fe (HS-Fe-PEG) NPs to produce HER2-targeted HS-Fe-PEG (HS-Fe-PEG-HER2) NPs.
The toxicity of HS-Fe-PEG-HER2 NPs on targeted cells in vitro and blood
and organ tissue of mice in vivo was investigated. Distribution in vivo
was also studied. Confocal laser-scanning microscopy and flow cytometry were
used to evaluate the targeting ability of HS-Fe-PEG-HER2 NPs in vitro. US
and MR instruments were used for imaging both in vivo and in vitro.
Results: The
obtained HS-Fe-PEG-HER2 NPs (average diameter 234.42±48.76 nm) exhibited good
physical properties and biosafety. In solution, they showed obvious enhancement
of the US signal and negative contrast in T 2-weighted MR imaging. The binding rate of
HS-Fe-PEG-HER2 NPs to targeted cells (SKBR3) was 78.97%±4.41% in vitro. US
and MR imaging in vivo confirmed that the HS-Fe-PEG-HER2 NPs were
delivered passively into the tumor region of SKBR3 and bound specifically to
tumor cells. Target enhancement was better than untargeted and targeted competition
groups.
Conclusion: HS-Fe-PEG-HER2
NPs have potential as a low-cytotoxicity and dual-mode US–MR-specific imaging
agent.
Keywords: dual-mode,
ultrasound imaging, magnetic resonance imaging, HER2, breast cancer