9 0 8 0 2
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 2.6 Breast Cancer (Dove Med Press)
- 3.9 Clin Epidemiol
- 3.3 Cancer Manag Res
- 3.9 Infect Drug Resist
- 3.6 Clin Interv Aging
- 4.8 Drug Des Dev Ther
- 2.8 Int J Chronic Obstr
- 8.0 Int J Nanomed
- 2.3 Int J Women's Health
- 3.2 Neuropsych Dis Treat
- 4.0 OncoTargets Ther
- 2.2 Patient Prefer Adher
- 2.8 Ther Clin Risk Manag
- 2.7 J Pain Res
- 3.3 Diabet Metab Synd Ob
- 4.3 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.9 Pharmgenomics Pers Med
- 3.5 Risk Manag Healthc Policy
- 4.5 J Inflamm Res
- 2.3 Int J Gen Med
- 4.1 J Hepatocell Carcinoma
- 3.2 J Asthma Allergy
- 2.3 Clin Cosmet Investig Dermatol
- 3.3 J Multidiscip Healthc

已发表论文
灵活剂量的棕榈酸帕利哌酮 (Paliperidone palmitate) 对中国急性精神分裂症患者的疗效及安全性: 一个非盲、单臂、前瞻性的介入性研究
Authors Si TM, Zhang KR, Tang JS, Fang MS, Li KQ, Zhuo JM, Feng Y
Published Date June 2015 Volume 2015:11 Pages 1483—1492
DOI http://dx.doi.org/10.2147/NDT.S81760
Received 28 January 2015, Accepted 30 March 2015, Published 22 June 2015
Approved for publication by Professor Wai Kwong Tang
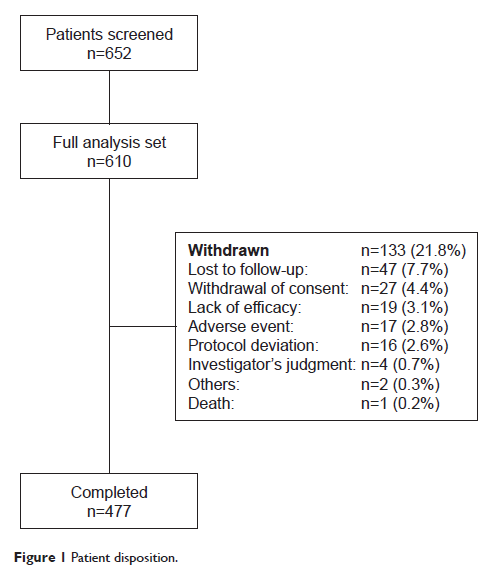
Abstract: This open-label, single-arm, multicenter, 13-week, prospective study explored the efficacy, safety, and tolerability of paliperidone palmitate (150 milligram equivalents [mg eq] [day 1], 100 mg eq [day 8], both deltoid injections; 75–150 mg eq, deltoid/gluteal injection) in Chinese patients with acute schizophrenia (Positive and Negative Syndrome Scale [PANSS] total score ≥70), who previously had unsatisfactory therapeutic effect following oral antipsychotic treatment (without washout period). Primary efficacy endpoint was percentage of patients with ≥30% improvement in the PANSS total score at the end of 13 weeks. Secondary efficacy endpoints included change from baseline to end of week 13 in PANSS total score, PANSS subscale scores, Marder factor scores, Clinical Global Impressions–Severity score, and Personal and Social Performance Scale scores. Overall, 477/610 enrolled patients (full analysis set, 78.2%) completed the study (men: 55.1%; women: 44.9%; mean age: 31.5 years). Total, 443/610 (72.6%, full analysis set) patients achieved primary endpoint (mean [standard deviation] change from baseline: –30.9 [19.51]). All secondary endpoints demonstrated significant improvement at the end of 13 weeks. One death occurred during this acute phase. The most common (>5%) treatment-emergent adverse events were extrapyramidal disorders (8.4%). The efficacy and safety data are consistent with other short-term, placebo-controlled studies of paliperidone palmitate conducted in similar populations.
Keywords: antipsychotic, long-acting injectable, PANSS
Keywords: antipsychotic, long-acting injectable, PANSS