9 0 9 6 8
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 2.6 Breast Cancer (Dove Med Press)
- 3.9 Clin Epidemiol
- 3.3 Cancer Manag Res
- 3.9 Infect Drug Resist
- 3.6 Clin Interv Aging
- 4.8 Drug Des Dev Ther
- 2.8 Int J Chronic Obstr
- 8.0 Int J Nanomed
- 2.3 Int J Women's Health
- 3.2 Neuropsych Dis Treat
- 4.0 OncoTargets Ther
- 2.2 Patient Prefer Adher
- 2.8 Ther Clin Risk Manag
- 2.7 J Pain Res
- 3.3 Diabet Metab Synd Ob
- 4.3 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.9 Pharmgenomics Pers Med
- 3.5 Risk Manag Healthc Policy
- 4.5 J Inflamm Res
- 2.3 Int J Gen Med
- 4.1 J Hepatocell Carcinoma
- 3.2 J Asthma Allergy
- 2.3 Clin Cosmet Investig Dermatol
- 3.3 J Multidiscip Healthc

SLC5A1 通过葡萄糖依赖性 AMPK/mTOR 信号传导促进胰腺癌的生长和增殖
Authors Gao HF, Chen LY, Cheng CS, Chen H, Meng ZQ, Chen Z
Received 21 November 2018
Accepted for publication 11 February 2019
Published 12 April 2019 Volume 2019:11 Pages 3171—3185
DOI https://doi.org/10.2147/CMAR.S195424
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Andrew Yee
Peer reviewer comments 2
Editor who approved publication: Dr Beicheng Sun
Background: Accumulating
studies have reported that aberrant expression of SLC5A1 is a negative
prognostic factor to various cancer patients.
Purpose: Pancreatic
cancer tissue has also shown to harbor higher expression of SLC5A1, however how
SLC5A1 mediates pancreatic cancer cells growth remains unclear.
Methods: In this study,
we examined the mRNA and protein expressions of SLC5A1 in human pancreatic
tissue and various cell lines. The in vitro and in vivo roles of SLC5A1 in
pancreatic cancer were investigated through stably transfected pancreatic cells
with shRNA plasmid targeting SLC5A1.
Results: Our results
observed SLC5A1 was over-expressed in human pancreatic cancer tissues as well
as most pancreatic cancer cell lines. Both in vitro and in vivo inhibition of
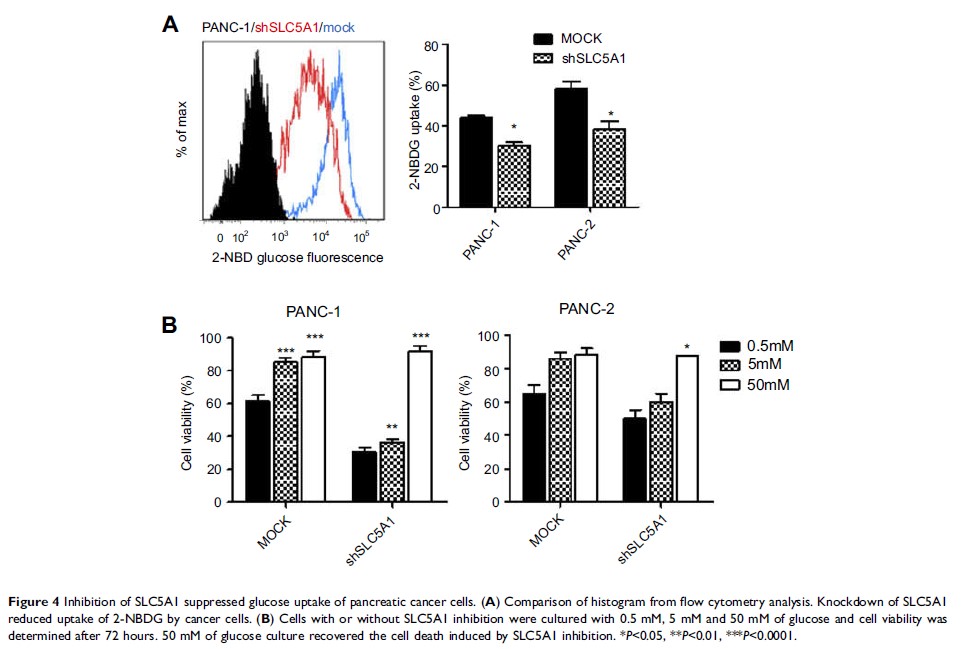
SLC5A1 retarded pancreatic cancer cell growth and progression. The SLC5A1
knockdown mediated growth suppression is mainly regulated by reduced cellular
glucose uptake by pancreatic cancer cells. Our further mechanistic observation
showed that inhibition of SLC5A1 induced AMPK-dependent mTOR suppression and
pharmacological inhibition of AMPK rescued the effect of SLC5A1 blockade.
Further protein-protein interaction analysis showed association of SLC5A1 with
EGFR and knockdown of EGFR also showed decreased cellular survival and glucose
uptake by pancreatic cancer cells.
Conclusion: Our findings
postulated SLC5A1/EGFR as the potential therapeutic target of pancreatic cancer
patients.
Keywords: pancreatic
cancer, SLC5A1, EGFR, cancer cell survival