9 0 8 0 2
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 2.6 Breast Cancer (Dove Med Press)
- 3.9 Clin Epidemiol
- 3.3 Cancer Manag Res
- 3.9 Infect Drug Resist
- 3.6 Clin Interv Aging
- 4.8 Drug Des Dev Ther
- 2.8 Int J Chronic Obstr
- 8.0 Int J Nanomed
- 2.3 Int J Women's Health
- 3.2 Neuropsych Dis Treat
- 4.0 OncoTargets Ther
- 2.2 Patient Prefer Adher
- 2.8 Ther Clin Risk Manag
- 2.7 J Pain Res
- 3.3 Diabet Metab Synd Ob
- 4.3 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.9 Pharmgenomics Pers Med
- 3.5 Risk Manag Healthc Policy
- 4.5 J Inflamm Res
- 2.3 Int J Gen Med
- 4.1 J Hepatocell Carcinoma
- 3.2 J Asthma Allergy
- 2.3 Clin Cosmet Investig Dermatol
- 3.3 J Multidiscip Healthc

负载顺铂 - 齐墩果酸的碳酸钙纳米粒对肝细胞癌细胞的协同作用可增强细胞凋亡和降低肝毒性
Authors Khan MW, Zhao P, Khan A, Raza F, Raza SM, Sarfraz M, Chen Y, Li M, Yang T, Ma X, Xiang G
Received 3 December 2018
Accepted for publication 19 March 2019
Published 28 May 2019 Volume 2019:14 Pages 3753—3771
DOI https://doi.org/10.2147/IJN.S196651
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Colin Mak
Peer reviewer comments 2
Editor who approved publication: Dr Lei Yang
Background: Cisplatin (CDDP), a widely used chemotherapeutic agent against hepatocellular carcinoma (HCC), faces severe resistance and hepatotoxicity problems which can be alleviated through combination therapy.
Purpose: The objective of this study was to develop a pH-dependent calcium carbonate nano-delivery system for the combination therapy of CDDP with oleanolic acid (OA).
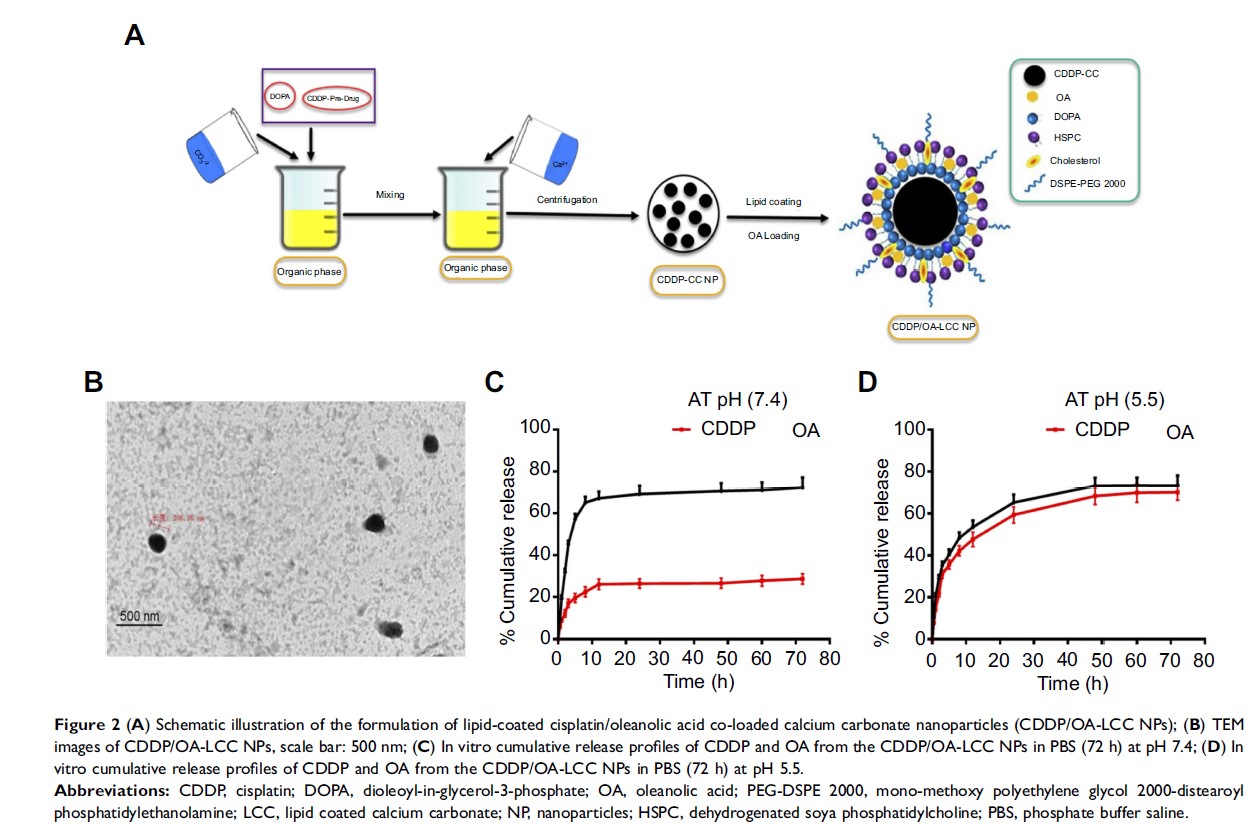
Methods: A microemulsion method was employed to generate lipid coated cisplatin/oleanolic acid calcium carbonate nanoparticles (CDDP/OA-LCC NPs), and the loading concentration of CDDP and OA was measured by atomic absorption spectroscopy and HPLC respectively.Transmission electron microscopy (TEM) was used to examine the nanoparticles morphology while its pH dependent release characteristics were investigated through in vitro release study. Cellular uptake was examined through a fluorescence microscopy. Apoptotic assays and western blot analysis were conducted to explore the synergistic apoptotic effect of OA on CDDP against HCC cells. The hepatoprotective of OA for CDDP was evaluated through H&E staining.
Results: TEM analysis revealed nanoparticles spherical shape with an average particle size of 206±15 nm, and the overall entrapment efficiency was 63.70%±3.9%. In vitro drug release study confirmed the pH-dependent property of the formulation, with the maximum CDDP release of 70%±4.6% at pH 5.5, in contrast to 28%±4.1% CDDP release at pH 7.4. Annexin V-FITC/PI assay and cell cycle analysis confirmed that CDDP and OA synergistically promoted greater HepG2 cells apoptosis for the CDDP/OA-LCC NPs as compared to their individual free drug solutions and NPs-treated groups. Western blot analysis also proved that CDDP/OA-LCC NPs induced the apoptosis by enhancing the proapoptotic protein expressions through downregulating P13K/AKT/mTOR pathway and upregulating p53 proapoptotic pathway. OA helped CDDP to overcome the resistance by downregulating the expression of proteins like XIAP, Bcl-2 via NF-κB pathway. OA also significantly alleviated CDDP-induced hepatotoxicity as evident from the decreased alanine transaminase, aspartate transaminase levels and histochemical evaluation. The possible mechanism may be related to the Nrf-2 induction via its antioxidant mechanism to maintain the redox balance and reduction in CYP2E1 activity which can lead to ROS-mediated oxidative stress.
Conclusion: These results suggest that CDDP/OA-LCC NPs have promising applications for co-delivering CDDP and OA to synergize their anti-tumor activity against HCC and to utilize OA’s protective effect against CDDP-induced hepatotoxicity.
Keywords: combination therapy, cisplatin, oleanolic acid, hepatocellular carcinoma, hepatotoxicity