9 0 8 1 0
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 2.6 Breast Cancer (Dove Med Press)
- 3.9 Clin Epidemiol
- 3.3 Cancer Manag Res
- 3.9 Infect Drug Resist
- 3.6 Clin Interv Aging
- 4.8 Drug Des Dev Ther
- 2.8 Int J Chronic Obstr
- 8.0 Int J Nanomed
- 2.3 Int J Women's Health
- 3.2 Neuropsych Dis Treat
- 4.0 OncoTargets Ther
- 2.2 Patient Prefer Adher
- 2.8 Ther Clin Risk Manag
- 2.7 J Pain Res
- 3.3 Diabet Metab Synd Ob
- 4.3 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.9 Pharmgenomics Pers Med
- 3.5 Risk Manag Healthc Policy
- 4.5 J Inflamm Res
- 2.3 Int J Gen Med
- 4.1 J Hepatocell Carcinoma
- 3.2 J Asthma Allergy
- 2.3 Clin Cosmet Investig Dermatol
- 3.3 J Multidiscip Healthc

服用抗细胞毒性 T 淋巴细胞相关蛋白 4 药物后的免疫相关不良事件:综合系统评价和荟萃分析
Authors Xu H, Tan P, Zheng X, Huang Y, Lin T, Wei Q, Ai J, Yang L
Received 28 November 2018
Accepted for publication 16 April 2019
Published 4 July 2019 Volume 2019:13 Pages 2215—2234
DOI https://doi.org/10.2147/DDDT.S196316
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Colin Mak
Peer reviewer comments 2
Editor who approved publication: Dr Qiongyu Guo
Objective: Administration of drugs targeting anti-cytotoxic T-lymphocyte-associated protein-4 (CTLA-4) is often associated with serious immune-related adverse events (irAEs). Here, we performed a comprehensive analysis of organ-specific irAEs and treatment-related hematologic abnormalities and musculoskeletal disorders resulting from anti-CTLA-4 treatment.
Materials and methods: PubMed, the Cochrane library, Web of Science, and ClinicalTrials.gov were searched for studies between January 1990 and March 2018 reporting AEs associated with anti-CTLA-4 therapies.
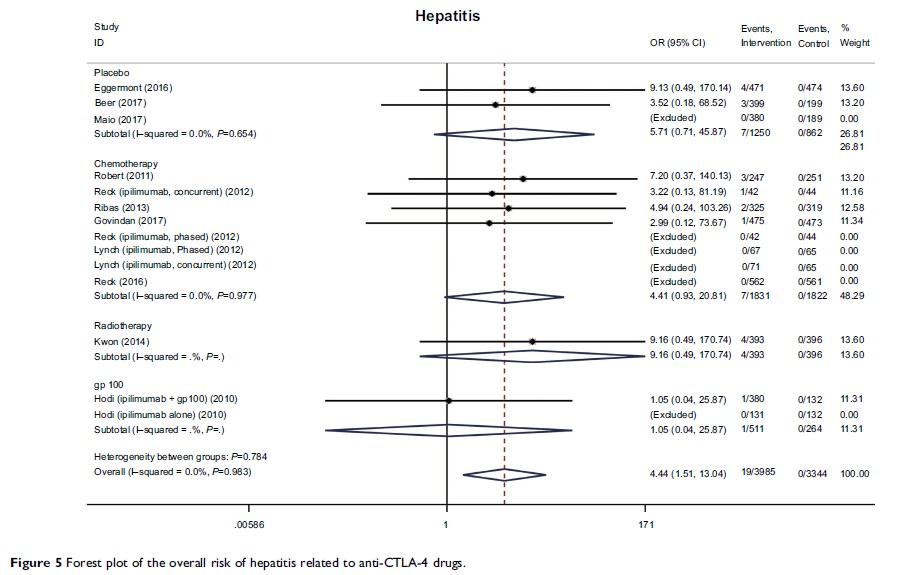
Results: A total of 11 clinical trials with 7,088 patients were included; of these, data were accessible for 10 on ClinicalTrials.gov. Compared with control therapies (placebo, chemotherapy, radiation therapy, or vaccine), anti-CTLA-4 therapies (ipilimumab and tremelimumab) were associated with an increased risk of serious irAEs, predominantly dermatologic (rash: odds ratio [OR] 3.39, P <0.01), gastrointestinal (diarrhea and colitis: OR 6.57 and 14.01, respectively; both P <0.001), endocrine (hypophysitis, hypothyroidism, adrenal insufficiency, and hypopituitarism: OR 4.22, 3.72, 3.77, and 4.73, respectively; all P <0.05), and hepatic (hepatitis, elevated alanine aminotransferase, and elevated aspartate aminotransferase: OR 4.44, 3.28, and 3.12, respectively; all P <0.05). The most common serious organ-specific irAEs were gastrointestinal (diarrhea 9.8% and colitis 5.3%). Although the incidence of selected events was higher in anti-CTLA-4-treated patients, no significant differences were found between anti-CTLA-4 and the control therapies in treatment-related hematologic abnormalities or severe musculoskeletal disorders.
Conclusion: Anti-CTLA-4 therapies are associated with an increased risk of serious organ-specific irAEs, most frequently involving the gastrointestinal system; however, no increased risk of hematologic abnormalities or severe musculoskeletal disorders was detected compared with other therapies. These results underscore the need for clinical awareness and prompt and effective management of multi-organ irAEs related to anti-CTLA-4 drugs.
Keywords: immune-related adverse events, anti-CTLA-4 drugs, ipilimumab, tremelimumab