9 1 2 3 6
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 2.6 Breast Cancer (Dove Med Press)
- 3.9 Clin Epidemiol
- 3.3 Cancer Manag Res
- 3.9 Infect Drug Resist
- 3.6 Clin Interv Aging
- 4.8 Drug Des Dev Ther
- 2.8 Int J Chronic Obstr
- 8.0 Int J Nanomed
- 2.3 Int J Women's Health
- 3.2 Neuropsych Dis Treat
- 4.0 OncoTargets Ther
- 2.2 Patient Prefer Adher
- 2.8 Ther Clin Risk Manag
- 2.7 J Pain Res
- 3.3 Diabet Metab Synd Ob
- 4.3 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.9 Pharmgenomics Pers Med
- 3.5 Risk Manag Healthc Policy
- 4.5 J Inflamm Res
- 2.3 Int J Gen Med
- 4.1 J Hepatocell Carcinoma
- 3.2 J Asthma Allergy
- 2.3 Clin Cosmet Investig Dermatol
- 3.3 J Multidiscip Healthc

利拉鲁肽通过 IRS2/PI3K/Akt 信号通路改善糖尿病小鼠的非酒精性脂肪性肝病
Authors Yang P, Liang Y, Luo Y, Li Z, Wen Y, Shen J, Li R, Zheng H, Gu HF, Xia N
Received 26 February 2019
Accepted for publication 16 April 2019
Published 4 July 2019 Volume 2019:12 Pages 1013—1021
DOI https://doi.org/10.2147/DMSO.S206867
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Ms Justinn Cochran
Peer reviewer comments 2
Editor who approved publication: Dr Juei-Tang Cheng
Purpose: High prevalence of nonalcoholic fatty liver disease (NAFLD) among patients with type 2 diabetes has implicated the role of hepatic insulin resistance (IR) in the diseases. To better understand the underlying mechanism, we have evaluated the pathophysiological effects of Liraglutide on NAFLD via the insulin signaling pathway.
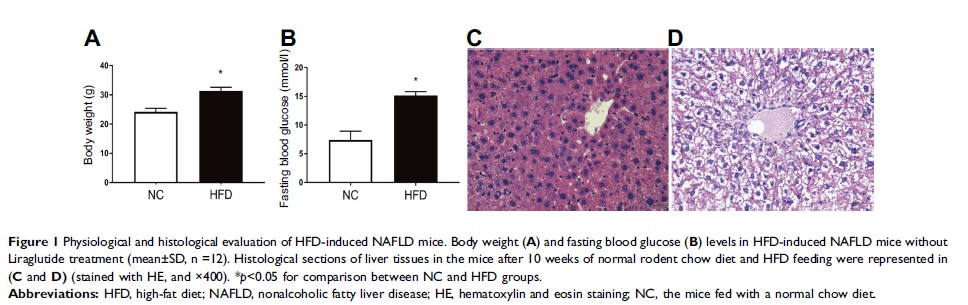
Patients and methods: A 2×2 factorial experiment was designed. High-fat diet (HFD)-induced NAFLD mice with diabetes were treated with Liraglutide for 10 weeks, while the control mice were saline-treated. Hepatic expressions of InsR, IGF-1R, IRS2, PI3K and Akt at mRNA and protein levels were analyzed with RT-PCR and Western blotting. Hematoxylin and eosin staining, Oil Red O staining and electron microscopy were used to visualize triglyceride accumulation in liver.
Results: Liraglutide significantly decreased body weight, fasting blood glucose levels and HOMA-IR scores in HFD mice. Compared with the control mice fed with chow diet, hepatic expressions of InsR, IRS2, PI3K and Akt at both mRNA and protein levels in HFD mice were significantly reduced, but upregulated after Liraglutide treatment. Furthermore, Liraglutide treatment was found to improve hepatic steatosis.
Conclusion: The current study thereby provides evidence that Liraglutide ameliorates NAFLD and improves hepatic steatosis mainly by upregulation of the IRS2/PI3K/Akt signaling mediators.
Keywords: glucagon like peptide 1, Liraglutide, nonalcoholic fatty liver disease, insulin resistance, insulin signaling