9 0 9 6 8
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 2.6 Breast Cancer (Dove Med Press)
- 3.9 Clin Epidemiol
- 3.3 Cancer Manag Res
- 3.9 Infect Drug Resist
- 3.6 Clin Interv Aging
- 4.8 Drug Des Dev Ther
- 2.8 Int J Chronic Obstr
- 8.0 Int J Nanomed
- 2.3 Int J Women's Health
- 3.2 Neuropsych Dis Treat
- 4.0 OncoTargets Ther
- 2.2 Patient Prefer Adher
- 2.8 Ther Clin Risk Manag
- 2.7 J Pain Res
- 3.3 Diabet Metab Synd Ob
- 4.3 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.9 Pharmgenomics Pers Med
- 3.5 Risk Manag Healthc Policy
- 4.5 J Inflamm Res
- 2.3 Int J Gen Med
- 4.1 J Hepatocell Carcinoma
- 3.2 J Asthma Allergy
- 2.3 Clin Cosmet Investig Dermatol
- 3.3 J Multidiscip Healthc

双 PI3K/mTOR 抑制剂 XL765 通过诱导 ER 应激依赖性细胞凋亡遏制胶质母细胞瘤生长
Authors Zhao H, Chen G, Liang H
Received 26 March 2019
Accepted for publication 21 June 2019
Published 8 July 2019 Volume 2019:12 Pages 5415—5424
DOI https://doi.org/10.2147/OTT.S210128
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Ms Rachel Predeepa
Peer reviewer comments 2
Editor who approved publication: Dr William Cho
Background: Deregulated phosphoinositide 3-kinase (PI3K)/mTOR signaling commonly exists in glioblastoma (GBM), making this axis an attractive target for therapeutic manipulation. A recent dual inhibitor of PI3K/mTOR pathway, XL765, exhibited an attractive suppression effect on GBM tumor growth. However, the exact functional mechanisms of tumor suppression mediated by XL765 have not yet been fully characterized.
Purpose: In this study, we took efforts to assess the effects of PI3K/mTOR blockade by XL765 on GBM growth in vitro and in vivo .
Methods: We analyzed the cytotoxicity of XL765 in three different GBM cell lines, A172, U87MG, and T98G, by using Hoechst 33258 (Invitrogen), Annexin V/propidium iodide (PI), as well as Cell Counting Kit -8 (CCK‐8) assay. We also used A172 xenograft model to study the effect of XL765 in vivo .
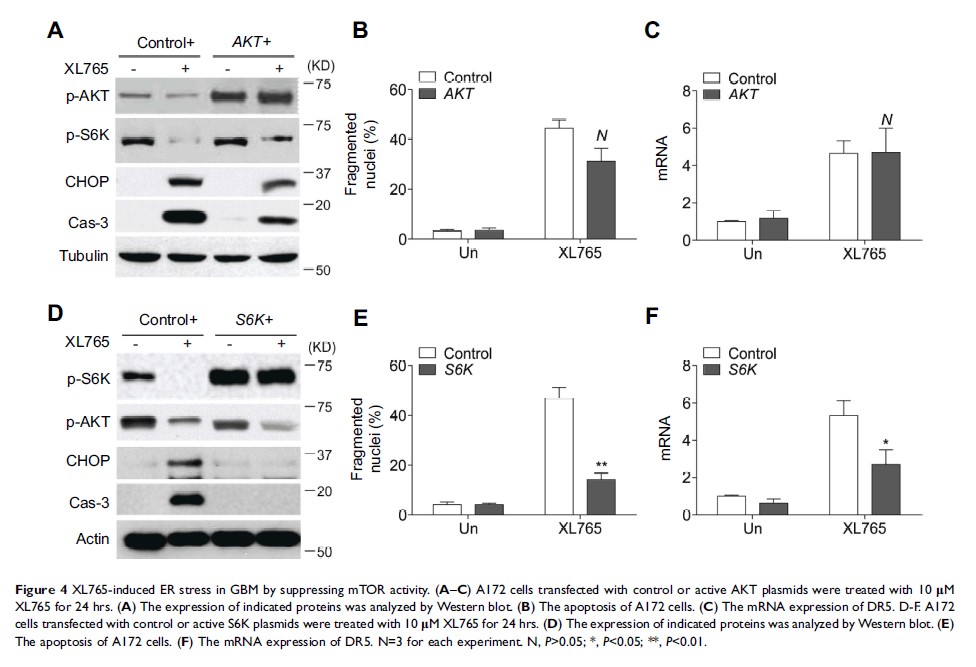
Results: We found that XL765 inhibits GBM viability with a wide range of potencies. Importantly, XL765 suppressed GBM cell growth by inducing endoplasmic reticulum (ER) stress dependent apoptosis. The activation of CHOP/DR5 pathway by XL765 induced ER stress is responsible for the induction of apoptosis. Moreover, the inhibition of mTOR signal by XL765 is the major source of ER stress, rather than inhibition of PI3K. At last, we demonstrated that combination of XL765 with GMB chemotherapeutic drug, temozolomide (TMZ), can achieved better therapy effect in vitro and in vivo .
Conclusion: Overall, our data show that targeting PI3K/mTOR by XL765 is a promising therapeutic strategy to relieve tumor burden in GBM patients.
Keywords: XL765, glioblastoma, apoptosis, ER stress