9 0 9 6 8
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 2.6 Breast Cancer (Dove Med Press)
- 3.9 Clin Epidemiol
- 3.3 Cancer Manag Res
- 3.9 Infect Drug Resist
- 3.6 Clin Interv Aging
- 4.8 Drug Des Dev Ther
- 2.8 Int J Chronic Obstr
- 8.0 Int J Nanomed
- 2.3 Int J Women's Health
- 3.2 Neuropsych Dis Treat
- 4.0 OncoTargets Ther
- 2.2 Patient Prefer Adher
- 2.8 Ther Clin Risk Manag
- 2.7 J Pain Res
- 3.3 Diabet Metab Synd Ob
- 4.3 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.9 Pharmgenomics Pers Med
- 3.5 Risk Manag Healthc Policy
- 4.5 J Inflamm Res
- 2.3 Int J Gen Med
- 4.1 J Hepatocell Carcinoma
- 3.2 J Asthma Allergy
- 2.3 Clin Cosmet Investig Dermatol
- 3.3 J Multidiscip Healthc

PD-L1 表达和分子分类的综合评估有助于胃癌的治疗选择和预后预测
Authors Sun Y, Yu W, Guan W, Cai L, Qiao M, Zheng L, Jiang R, Wang R, Wang L
Received 21 February 2019
Accepted for publication 20 May 2019
Published 10 July 2019 Volume 2019:11 Pages 6397—6410
DOI https://doi.org/10.2147/CMAR.S206189
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Melinda Thomas
Peer reviewer comments 2
Editor who approved publication: Dr Kenan Onel
Purpose: Targeting the PD-1/PD-L1 pathway has emerged as a novel therapy for cancer. To identify rational candidates for anti-PD-1/PD-L1 immunotherapy in gastric cancer (GC), the abundance of PD-L1 expression was evaluated on a kind of biomarker-based molecular classification for shaping prognosis and treatment planning.
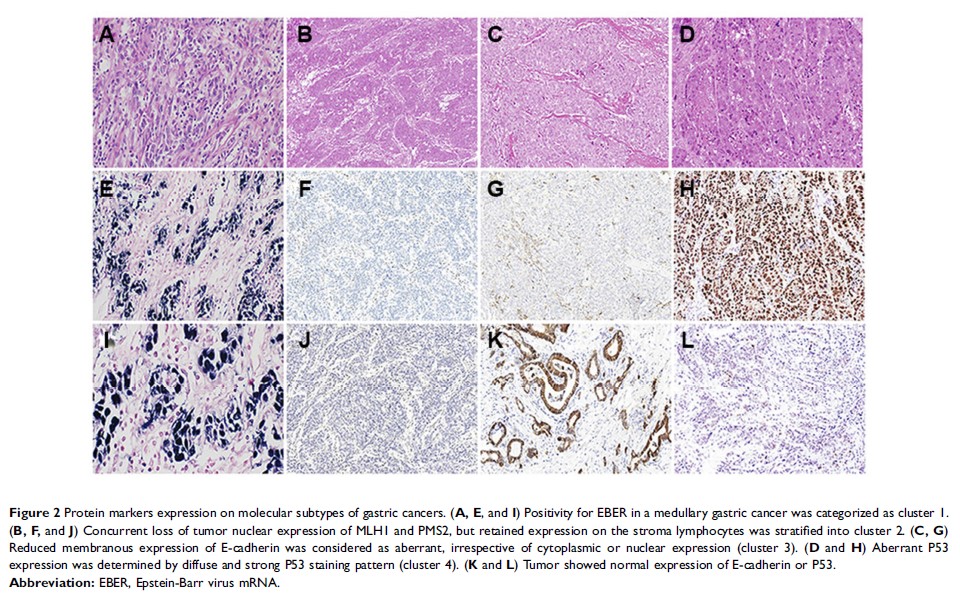
Methods: One hundred and sixty-five GCs were classified into five subgroups using immunohistochemistry (IHC) and in situ hybridization (ISH) methods, based on a panel of seven markers (MLH1, PMS2, MSH2, MSH6, E-cadherin, P53, and Epstein-Barr virus mRNA). The expression of PD-L1 in GC tissues was analyzed immunohistochemically.
Results: The five categories (Epstein–Barr virus positivity, microsatellite instability, aberrant E-cadherin, aberrant P53 expression, and normal P53 expression) correspond to the reported molecular subgroups for similar proportions and clinicopathologic characteristics. Survival analysis indicated that subgroups with aberrant E-cadherin expression independently predicted a worse prognosis in GC patients (HR=2.51, P =0.010). The clinical and prognostic profiles produced by this stratification in nonintestinal-type GC were distinguishable from those in intestinal-type. Although PD-L1 was not a significant prognostic factor, that more frequent presence of PD-L1-positive in microsatellite instability tumors than other subtypes (P <0.010) hinted at a prolonged clinical course. Moreover, the lowest level of PD-L1 but the highest of Her2 was observed in the group of aberrant P53, namely it was suggested that there was a negative correlation between PD-L1 and Her2 overexpression.
Conclusion: Different molecular subtypes in GC may have a tendency to react differently to anti-PD-L1/PD-1 immunotherapy or anti-Her2 therapy. A combination of PD-L1 expression and this cost-effective classification strategy would be helpful for predicting prognosis and promoting personalized therapy in clinical practice.
Keywords: PD-L1, molecular classification, gastric cancer, immunohistochemistry, in situ hybridization