9 1 2 3 6
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 2.6 Breast Cancer (Dove Med Press)
- 3.9 Clin Epidemiol
- 3.3 Cancer Manag Res
- 3.9 Infect Drug Resist
- 3.6 Clin Interv Aging
- 4.8 Drug Des Dev Ther
- 2.8 Int J Chronic Obstr
- 8.0 Int J Nanomed
- 2.3 Int J Women's Health
- 3.2 Neuropsych Dis Treat
- 4.0 OncoTargets Ther
- 2.2 Patient Prefer Adher
- 2.8 Ther Clin Risk Manag
- 2.7 J Pain Res
- 3.3 Diabet Metab Synd Ob
- 4.3 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.9 Pharmgenomics Pers Med
- 3.5 Risk Manag Healthc Policy
- 4.5 J Inflamm Res
- 2.3 Int J Gen Med
- 4.1 J Hepatocell Carcinoma
- 3.2 J Asthma Allergy
- 2.3 Clin Cosmet Investig Dermatol
- 3.3 J Multidiscip Healthc

化疗和厄洛替尼的间插联合疗法用于 IIIA 期非小细胞肺癌:一个多中心、开放标签、单臂 II 期研究
Authors Chen Z, Shen S, Shi W, Jiang G, Wang X, Jian H, Zhou Z, Ding Z, Lu S
Received 30 September 2018
Accepted for publication 10 April 2019
Published 12 July 2019 Volume 2019:11 Pages 6543—6552
DOI https://doi.org/10.2147/CMAR.S189287
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Colin Mak
Peer reviewer comments 2
Editor who approved publication: Dr Ahmet Emre Eskazan
Objective: This multicenter, open-label, single-arm, phase II trial evaluated the efficacy and safety of an intercalated combination of erlotinib and gemcitabine/cisplatin or carboplatin in patients with stage IIIA non-small-cell lung cancer (NSCLC).
Registration: This trial is registered with ClinicalTrials.gov, number NCT01297101.
Methods: The primary endpoint was the objective response rate (ORR), which includes complete response (CR) and partial response (PR), assessed using RECIST version 1.0 in the intention-to-treat population. Adverse events (AEs) were graded by the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. Secondary endpoints included the disease control rate, disease-free survival (DFS), overall survival (OS), and safety. Between April 1, 2011, and July 31, 2014, 39 patients with stage IIIA NSCLC received two cycles of intercalated use of erlotinib with gemcitabine/cisplatin or carboplatin.
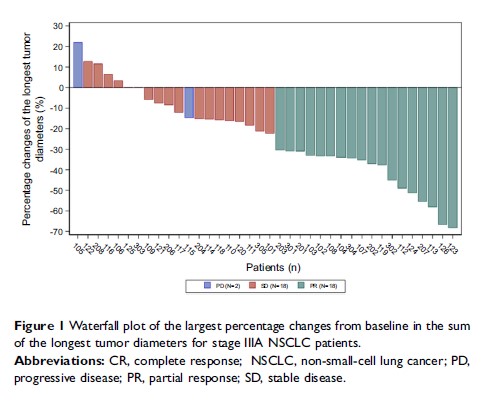
Results: Eighteen patients (46.15%) achieved a PR and no patient achieved a pathologic CR, resulting in an ORR of 46.15% (95% CI 30–63%). Median DFS was 20 months (95% CI 5.26–50.61) and median OS was 25 months (95% CI 15.57–33.39). Patients with EGFR mutations (n=7) had a higher ORR than those with wild-type EGFR (n=9) (85.71% vs 55.56%, P=0.00). Most AEs were CTCAE grade 1 or 2; there were no cases of increased hematologic toxicity or erlotinib-emergent interstitial lung disease observed.
Conclusion: Two cycles of intercalated neoadjuvant therapy with erlotinib and gemcitabine/cisplatin or carboplatin were effective and safe for patients with stage IIIA NSCLC. This approach should be further explored in larger randomized controlled trials given the lack of a consensus about the best treatment for stage IIIA NSCLC.
Keywords: non-small-cell lung cancer, NSCLC, neoadjuvant, erlotinib, gemcitabine, platinum, objective response rate, progression-free survival, overall survival