111314
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

基于肿瘤细胞泛素化蛋白的纳米疫苗内置佐剂的抗肿瘤功效
Authors Huang F, Zhao J, Wei Y, Wen Z, Zhang Y, Wang X, Shen Y, Wang L, Pan N
Received 6 November 2019
Accepted for publication 26 January 2020
Published 13 February 2020 Volume 2020:15 Pages 1021—1035
DOI https://doi.org/10.2147/IJN.S237578
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 3
Editor who approved publication: Dr Mian Wang
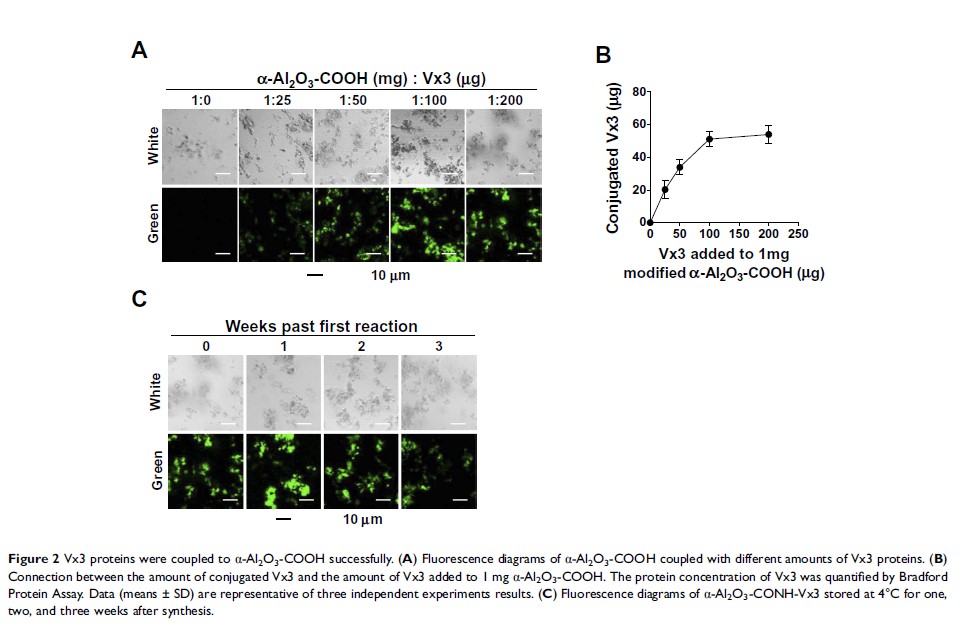
Background and Aim: We have previously identified ubiquitinated proteins (UPs) from tumor cell lysates as a promising vaccine for cancer immunotherapy in different mouse tumor models. In this study, we aimed at developing a highly efficient therapeutic adjuvant built-in nanovaccine (α-Al2O3-UPs) by a simple method, in which UPs from tumor cells could be efficiently and conveniently enriched by α-Al2O3 nanoparticles covalently coupled with Vx3 proteins (α-Al2O3-CONH-Vx3).
Methods: The α-Al2O3 nanoparticles were modified with 4-hydroxybenzoic acid followed by coupling with ubiquitin-binding protein Vx3. It was then used to enrich UPs from 4T1 cell lysate. The stability and the efficiency for the UPs enrichment of α-Al2O3-CONH-Vx3 were examined. The ability of α-Al2O3-UPs to activate DCs was examined in vitro subsequently. The splenocytes from the vaccinated mice were re-stimulated with inactivated tumor cells, and the IFN-γ secretion was detected by ELISA and flow cytometry. Moreover, the therapeutic efficacy of α-Al2O3-UPs, alone and in combination with chemotherapy, was examined in 4T1 tumor-bearing mice.
Results: Our results showed that α-Al2O3-UPs were successfully synthesized and abundant UPs from tumor cell lysate were enriched by the new method. In vitro study showed that compared to the physical mixture of α-Al2O3 nanoparticles and UPs (α-Al2O3+UPs), α-Al2O3-UPs stimulation resulted in higher upregulations of CD80, CD86, MHC class I, and MHC class II on DCs, indicating the higher ability of DC activation. Moreover, α-Al2O3-UPs elicited a more effective immune response in mice, demonstrated by higher IFN-γ secretion than α-Al2O3+UPs. Furthermore, α-Al2O3-UPs also exhibited a more potent effect on tumor growth inhibition and survival prolongation in 4T1 tumor-bearing mice. Notably, when in combination with low dose chemotherapy, the anti-tumor effect was further enhanced, rather than using α-Al2O3-UPs alone.
Conclusion: This study presents an adjuvant built-in nanovaccine generated by a new simple method that can be potentially applied to cancer immunotherapy and lays the experimental foundation for future clinical application.
Keywords: ubiquitinated proteins, alumina nanoparticles, cancer vaccine, combination therapy