111314
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

对中国健康志愿者进行单次和多次头孢替坦二钠静脉给药的药代动力学和耐受性
Authors Liu J, Zhai Y, Wu L, Wu G, Zheng Y, Hu X, Shentu J
Received 16 October 2019
Accepted for publication 29 January 2020
Published 13 February 2020 Volume 2020:14 Pages 613—620
DOI https://doi.org/10.2147/DDDT.S234619
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 2
Editor who approved publication: Dr Qiongyu Guo
Background: Cefotetan is highly stable to penicillinase and cephalosporin produced by gram-negative bacteria, and it has strong antimicrobial activity against most gram-negative bacteria, some anaerobic bacteria and streptococcus. The objective of this study was to evaluate the pharmacokinetic profile and tolerability of single and multiple intravenous doses of cefotetan disodium in healthy Chinese volunteers.
Methods: In this single-center, open-label, dose-escalating study, subjects were randomized to receive a single dose of cefotetan disodium 0.5, 1.0, or 2.0 g administered as a 1 h intravenous infusion. After completion of the single-dose phase, subjects continued into the multiple-dose phase, in which they received 1.0 g cefotetan disodium BID for 7 consecutive days. Plasma samples were assayed by a validated high-performance liquid chromatography-tandem mass spectrometry method. Pharmacokinetic parameters were calculated and analyzed statistically. Tolerability was assessed based on physical examinations, vital signs, laboratory tests, and subject interviews.
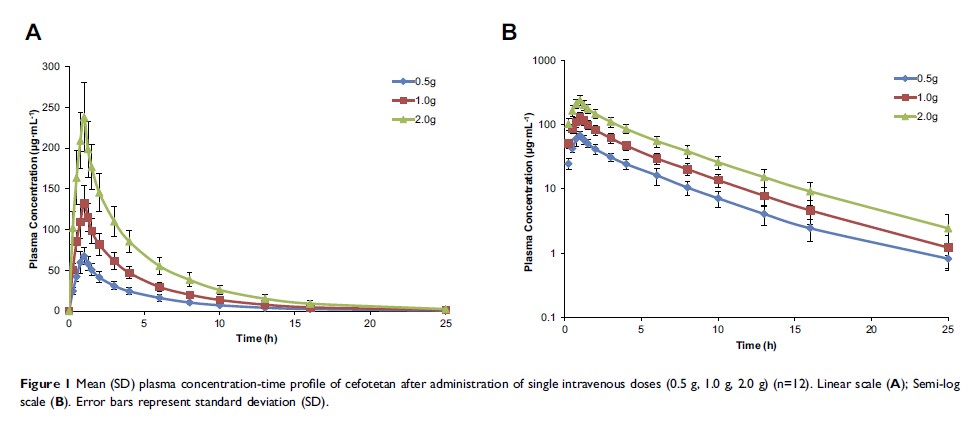
Results: After intravenous administration of single doses of 0.5, 1.0, and 2.0 g cefotetan disodium, the pharmacokinetics of cefotetan were as follows: Cmax was 69.49± 12.10 μg·mL− 1, 132.03± 22.56 μg·mL− 1 and 237.75± 42.12 μg·mL− 1, respectively; AUClast was 278.29± 51.13 μg·mL− 1·h, 543.25± 92.44 μg·mL− 1·h and 1003.8± 172.39 μg·mL− 1·h, respectively; AUC∞ was 284.42± 50.76 μg·mL− 1·h, 551.38± 95.83 μg·mL− 1·h and 1020.18± 181.19 μg·mL− 1·h, respectively; t1/2 was 4.21± 0.83 h, 4.39± 0.53 h and 4.27± 0.74 h, respectively; CL was 1.81± 0.33 L·h− 1, 1.86± 0.32 L·h− 1 and 2.02± 0.38 L·h− 1, respectively; Vd was 10.80± 1.89L, 11.78± 2.20L and 12.25± 1.99L, respectively. In the multiple-dose study, the pharmacokinetics of cefotetan were as follows: Cmax,ss was 147.58± 22.71 μg·mL− 1; Cmin,ss was 12.92± 3.70 μg·mL− 1; Cavg was 45.10± 7.78 μg·mL− 1; AUCτ,ss was 541.15± 93.36 μg·mL− 1·h; AUC∞ was 612.06± 114.23 μg·mL− 1·h; t1/2 was 4.30± 0.63 h; CL was 1.90± 0.35L·h− 1; Vd was 8.91± 1.57L; DF was 300.92± 33.28%; Accumulation Index was 1.17± 0.05. No serious adverse events were reported. Adverse events were generally mild.
Conclusion: Cefotetan disodium showed favorable tolerability in this study. The Cmax and AUCs of cefotetan disodium demonstrated dose-dependent pharmacokinetic characteristics after single dose over a dose range (0.5– 2.0 g) in healthy subjects, whereas the t1/2 was independent of dose. Except for Vd, there was no difference in other pharmacokinetic parameters between multiple and single administration.
Keywords: cefotetan disodium, pharmacokinetics, healthy volunteers, plasma, single-dose, multiple-dose