111314
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

包含尼莫地平药物溶液和纳米乳剂的新型药物递送载体:制备、表征、体外和体内研究
Authors Huang S, Huang Z, Fu Z, Shi Y, Dai Q, Tang S, Gu Y, Xu Y, Chen J, Wu X, Ren F
Received 9 August 2019
Accepted for publication 3 February 2020
Published 18 February 2020 Volume 2020:15 Pages 1161—1172
DOI https://doi.org/10.2147/IJN.S226591
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 2
Editor who approved publication: Dr Thomas Webster
Purpose: Nimodipine (NIMO) is used clinically to treat ischemic damage resulting from subarachnoid hemorrhage. However, clinical application of NIMO is limited by poor aqueous solubility and low safety. To overcome these limitations, a novel two-vial NIMO-loaded nanoemulsion (NIMO-TNE) was designed in this study.
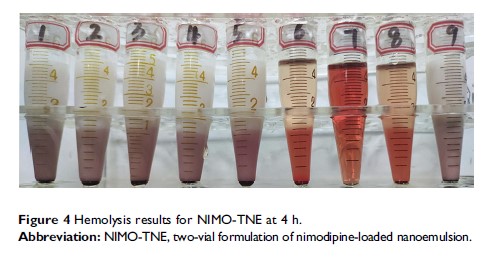
Methods: NIMO-TNE was prepared by mixing a nimodipine-polyethylene glycol 400 (NIMO-PEG400) solution and a commercially available 20% injectable blank nanoemulsion (BNE). Drug distribution in NIMO-TNE, physical stability, and dilution stability were evaluated in vitro, and pharmacokinetics and pharmacodynamics were evaluated in vivo. Safety was assessed using the hemolysis test and the intravenous irritation test, and acute toxicity of NIMO-TNE was compared with that of commercial Nimotop injection.
Results: Drug loading (DL) in NIMO-TNE was enhanced 5-fold compared with that in Nimotop injection. The mean particle size of NIMO-TNE was 241.53 ± 1.48 nm. NIMO-TNE and NIMO-TNE diluted in 5% glucose injection and 0.9% sodium chloride was stable for a sufficient duration to allow for clinical use. In addition, NIMO-TNE exhibited a similar pharmacokinetic profile and similar brain ischemia reduction in a rat middle cerebral artery occlusion (MCAO) model compared to Nimotop injection. Furthermore, NIMO-TNE did not induce hemolysis at 37°C, and NIMO-TNE induced less intravenous irritation than Nimotop injection. Moreover, NIMO-TNE could be injected at a 23-fold higher dose than the LD 50 of Nimotop injection with no obvious toxicity or side effects.
Conclusion: NIMO-TNE is a promising formulation suitable for intravenous injection, is easy to prepare, and exhibits excellent safety.
Keywords: nimodipine, nanoemulsion, pharmacokinetics, MCAO, LD50