111314
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

负载沙利霉素的明胶酶响应性纳米颗粒通过靶向药物递送和抑制宫颈癌干细胞发挥增强和延长的抗肿瘤作用
Authors Wang Q, Liu F, Wang L, Xie C, Wu P, Du S, Zhou S, Sun Z, Liu Q, Yu L, Liu B, Li R
Received 15 October 2019
Accepted for publication 26 January 2020
Published 26 February 2020 Volume 2020:15 Pages 1283—1295
DOI https://doi.org/10.2147/IJN.S234679
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 3
Editor who approved publication: Dr Mian Wang
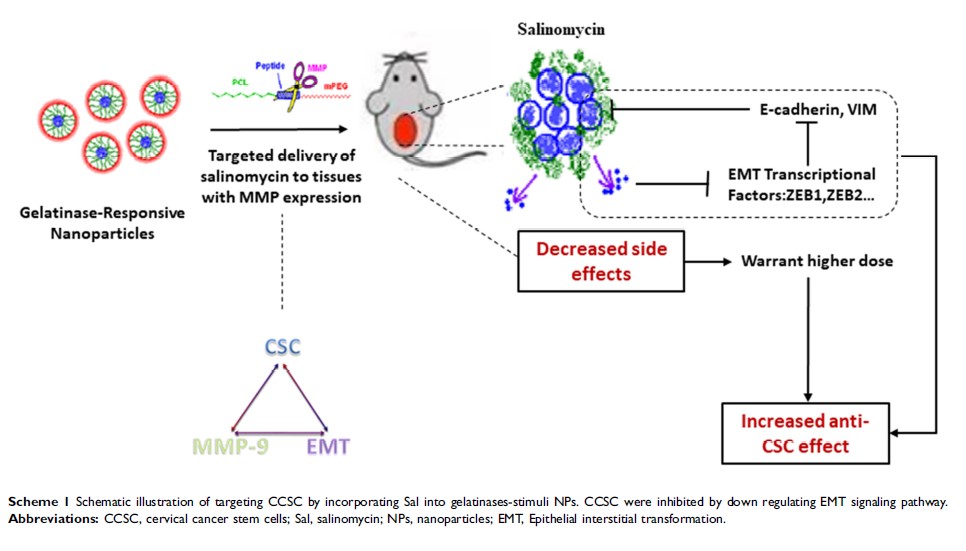
Background: Cervical cancer stem cells (CCSCs) represent a subpopulation of tumor cells that possess self-renewal capacity and numerous intrinsic mechanisms of resistance to conventional chemotherapy and radiotherapy. These cells play a crucial role in relapse and metastasis of cervical cancer. Therefore, eradication of CCSCs is the primary objective in cervical cancer therapy. Salinomycin (Sal) is an agent used for the elimination of cancer stem cells (CSCs); however, the occurrence of several side effects hinders its application. Nanoscale drug-delivery systems offer great promise for the diagnosis and treatment of tumors. These systems can be used to reduce the side effects of Sal and improve clinical benefit.
Methods: Sal-loaded polyethylene glycol-peptide-polycaprolactone nanoparticles (Sal NPs) were fabricated under mild and non-toxic conditions. The real-time biodistribution of Sal NPs was investigated through non-invasive near-infrared fluorescent imaging. The efficacy of tumor growth inhibition by Sal NPs was evaluated using tumor xenografts in nude mice. Flow cytometry, immunohistochemistry, and Western blotting were used to detect the apoptosis of CSCs after treatment with Sal NPs. Immunohistochemistry and Western blotting were used to examine epithelial–mesenchymal transition (epithelial interstitial transformation) signal-related molecules.
Results: Sal NPs exhibited antitumor efficacy against cervical cancers by inducing apoptosis of CCSCs and inhibiting the epithelial–mesenchymal transition pathway. Besides, tumor pieces resected from Sal NP-treated mice showed decreased reseeding ability and growth speed, further demonstrating the significant inhibitory ability of Sal NPs against CSCs. Moreover, owing to targeted delivery based on the gelatinase-responsive strategy, Sal NPs was more effective and tolerable than free Sal.
Conclusion: To the best of our knowledge, this is the first study to show that CCSC-targeted Sal NPs provide a potential approach to selectively target and efficiently eradicate CCSCs. This renders them a promising strategy to improve the therapeutic effect against cervical cancer.
Keywords: nanoparticles, salinomycin, tumor-targeted delivery, cancer stem cells, epithelial interstitial transformation