111314
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

运甲状腺素蛋白淀粉状蛋白生成抑制剂的结构和活性综述
Authors Guo X, Liu Z, Zheng Y, Li Y, Li L, Liu H, Chen Z, Wu L
Received 4 November 2019
Accepted for publication 24 February 2020
Published 10 March 2020 Volume 2020:14 Pages 1057—1081
DOI https://doi.org/10.2147/DDDT.S237252
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 4
Editor who approved publication: Dr Tuo Deng
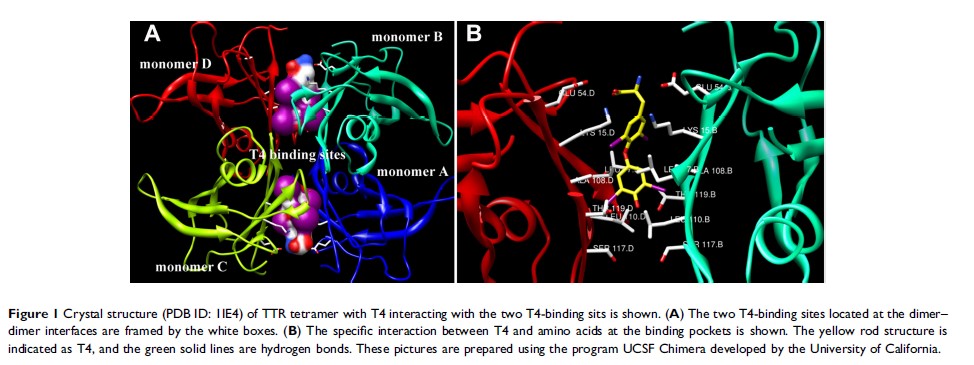
Abstract: Transthyretin (TTR) is a tetrameric protein, and its dissociation, aggregation, deposition, and misfolding are linked to several human amyloid diseases. As the main transporter for thyroxine (T4) in plasma and cerebrospinal fluid, TTR contains two T4-binding sites, which are docked with T4 and subsequently maintain the structural stability of TTR homotetramer. Affected by genetic disorders and detrimental environmental factors, TTR degrades to monomer and/or form amyloid fibrils. Reasonably, stabilization of TTR might be an efficient strategy for the treatment of TTR-related amyloidosis. However, only 10– 25% of T4 in the plasma is bound to TTR under physiological conditions. Expectedly, T4 analogs with different structures aiming to bind to T4 pockets may displace the functions of T4. So far, a number of compounds including both natural and synthetic origin have been reported. In this paper, we summarized the potent inhibitors, including bisaryl structure-based compounds, flavonoids, crown ethers, and carboranes, for treating TTR-related amyloid diseases and the combination modes of some compounds binding to TTR protein.
Keywords: transthyretin, amyloidogenesis, stabilization, inhibitors