111314
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

右旋美托咪定术中输注和术后 PCIA 对剖宫产术后早期母乳喂养的影响:一项随机双盲对照试验
Authors Wang Y, Fang X, Liu C, Ma X, Song Y, Yan M
Received 4 December 2019
Accepted for publication 24 February 2020
Published 11 March 2020 Volume 2020:14 Pages 1083—1093
DOI https://doi.org/10.2147/DDDT.S241153
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Objective: Few studies have investigated the effects of dexmedetomidine (DEX) on breastfeeding after cesarean delivery. A randomized double-blind controlled trial was conducted to investigate whether the administration of DEX, immediately after delivery and for patient-controlled intravenous analgesia (PCIA), can be beneficial for breastfeeding.
Patients and Methods: One hundred sixty parturients scheduled for elective cesarean section under spinal anesthesia were randomly allocated to the DEX group (a loading dose of DEX was pumped at 0.5 μg/kg within 10 min, followed by a further infusion of DEX at 0.5 μg/kg/h until the end of the surgery and PCIA for 2 days with DEX plus sufentanil) or the standard care group (infusion saline intraoperatively, and PCIA for 2 days with sufentanil). The number of days required to switch to exclusive breastfeeding within six weeks of delivery, the time to first lactation and breast milk volume on day 1 and day 2 after delivery were recorded. Recovery quality, comfort, anxiety, depression, postoperative analgesia, and adverse reactions of parturients were also assessed.
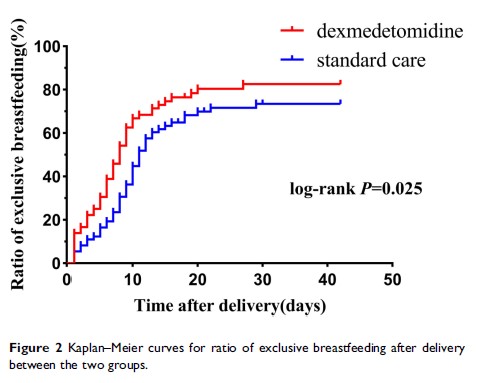
Results: Compared with the standard care group, parturients in the DEX group could be converted to exclusive breastfeeding earlier (11 [14] vs 8 [10] days, log-rank P=0.025), the first lactation time was sooner (28.38 [13.82] vs 33.79 [14.85] hrs, P=0.024), and the amount of breast milk on the second day after delivery increased (P=0.012). There was no difference between the two groups in postpartum uterine contraction pain, but postpartum rest and movement VAS scores and recovery quality score in the DEX group were better than those in the standard care group (all P< 0.05). Moreover, the hospital anxiety and depression scale and anxiety subscale score on the second day after delivery and the comfort score on the third day after delivery in the DEX group were significantly better than those in the standard care group (5 [5] vs 6 [8], 2 [2] vs 3 [3], 83.58 [6.75] vs 80.48 [6.58]; P=0.013, P=0.005, P=0.006, respectively). The incidence of adverse events, such as bradycardia, vomiting, hypersomnia, hypertension and hypotension, was not significantly different between the DEX and standard care groups (6.9% vs 2.7%, 5.6% vs 13.7%, 4.2% vs 0%, 5.6% vs 2.7%, 11.1% vs 8.2%; P=0.275, P=0.158, P=0.366, P=0.681, P=0.556, respectively), except more parturients experienced nausea in the standard care group than in the DEX group (28.8% vs 11.1%, P=0.012). Furthermore, there was no difference in Neonatal Behavioral Neurological Assessment scores on the first and second days after delivery between the DEX and standard care groups (38 [3] vs 37 [2], 38.5 [2] vs 38 [2]; P=0.173, P=0.312, respectively).
Conclusion: The application of DEX in the perioperative period of cesarean section was not only conducive to the early conversion of infant feeding to exclusive breastfeeding but could also improve the recovery quality and comfort of the parturient, optimize analgesia, shorten the time to first lactation, and increase lactation.
Clinical Trials Registration: NCT03805945.
Keywords: dexmedetomidine, breastfeeding, cesarean section, intraoperative infusion, postoperative PCIA