111314
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

作为术后恶心和呕吐的用药,雷莫司琼与咪达唑仑在 0.9% 氯化钠注射液中的稳定性和相容性
Authors Xia J, Chen P
Received 1 January 2020
Accepted for publication 3 February 2020
Published 17 March 2020 Volume 2020:14 Pages 1169—1176
DOI https://doi.org/10.2147/DDDT.S244439
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 2
Editor who approved publication: Dr Georgios D. Panos
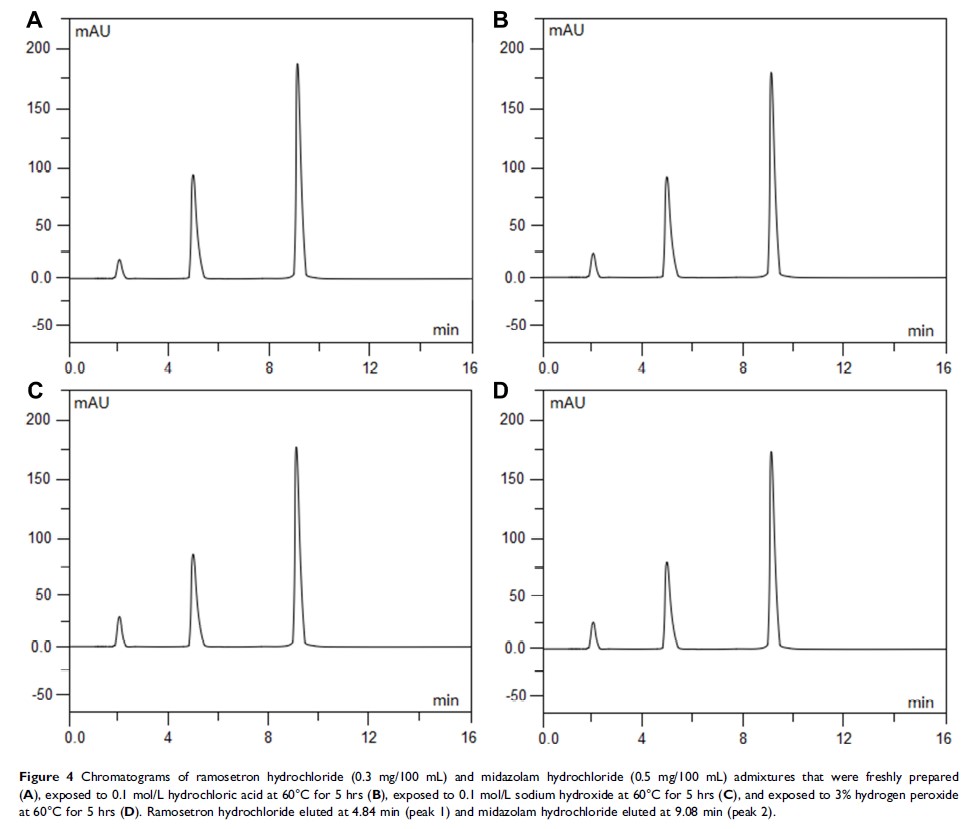
Background: Combination antiemetic therapy has become a common practice for the prevention of postoperative nausea and vomiting (PONV). The aim of the present study was to evaluate the stability and compatibility of ramosetron hydrochloride and midazolam in 0.9% sodium chloride injection when stored at 4°C and 25°C for up to 14 days.
Methods: Admixtures were assessed initially and for 14 days after preparation in polyolefin bags and glass bottles using 0.9% sodium chloride injection as the diluent and stored at 4°C or 25°C. The initial concentrations were 0.3 mg/100 mL ramosetron hydrochloride and 0.5 mg/100 mL midazolam hydrochloride. For all samples, the compatibility parameters (including precipitation, cloudiness, discoloration and pH values) were evaluated. Chemical stability was also determined using high-performance liquid chromatography (HPLC) analysis.
Results: After a 14-day period of storage at 4°C or 25°C, the percent of the initial concentration of ramosetron hydrochloride and midazolam hydrochloride in the various solutions were maintained at a minimum of 97%. All of the mixtures remained clear and colourless throughout the observation period, and no colour change or precipitation was observed.
Conclusion: The results indicate that admixtures of 0.3 mg/100 mL ramosetron hydrochloride and 0.5 mg/100 mL midazolam hydrochloride in normal saline were stable for 14 days at 4°C or 25°C when packaged in polyolefin bags or glass bottles and protected from light.
Keywords: ramosetron, midazolam, drug stability, postoperative nausea and vomiting