111314
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

在澳大利亚的晚期或转移性癌症患者中进行卡瑞利珠单抗首次用于人类的剂量研究
Authors Lickliter JD, Gan HK, Voskoboynik M, Arulananda S, Gao B, Nagrial A, Grimison P, Harrison M, Zou J, Zhang L, Luo S, Lahn M, Kallender H, Mannucci A, Somma C, Woods K, Behren A, Fernandez-Penas P, Millward M, Meniawy T
Received 26 December 2019
Accepted for publication 20 February 2020
Published 18 March 2020 Volume 2020:14 Pages 1177—1189
DOI https://doi.org/10.2147/DDDT.S243787
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 2
Editor who approved publication: Dr Tin Wui Wong
Purpose: Camrelizumab inhibits PD-1 in non-clinical models and showed typical non-clinical pharmacokinetic (PK) and safety profiles for an IgG4 monoclonal antibody. We report results from the First-in-Human Phase 1 trial of camrelizumab in Australian population.
Methods: Camrelizumab was administered to patients with advanced solid tumors who had failed standard therapies. In the dose-escalation phase (n=23), camrelizumab was administered intravenously at 1 mg/kg, 3 mg/kg, 6 mg/kg, and 10 mg/kg every 2 weeks. In dose expansion (n=26), camrelizumab was given at 200 mg or 600 mg every 4 weeks.
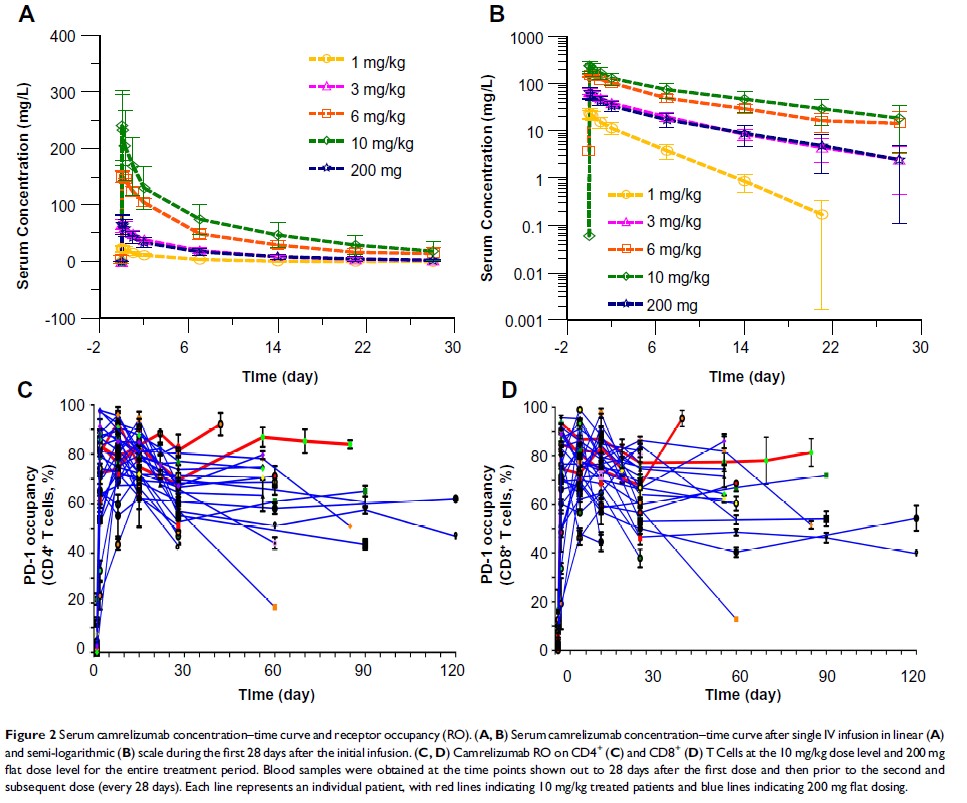
Results: Two dose-limiting toxicities were observed during dose escalation: transaminase elevation and diarrhea (both grade 3). Overall, treatment-related adverse events were consistent with the expected toxicity profile of immune checkpoint inhibition, with the striking exception of the dose-related development of angiomatous skin lesions characterized as reactive cutaneous capillary endothelial proliferation. The PK profile showed a dose-progressive increase in half-life from 3 days at 1 mg/kg to 7 days at 10 mg/kg. Moreover, receptor occupancy assays showed a PD-1 occupancy of > 50% in most patients out to 28 days post-dose. The objective response rate was 15.2% (95% CI 6.3– 28.9).
Conclusion: Camrelizumab has manageable toxicity and encouraging preliminary antitumor activity in advanced solid tumors in Australia.
Clinical Trial Registration: ClinicalTrials.gov Identifier: NCT02492789.
Keywords: PD-1, monoclonal antibody, first-in-human dose study, cancer, reactive cutaneous capillary endothelial proliferation