111314
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

阿瑞匹坦对健康中国人和高加索人的药代动力学比较
Authors Yang MJ, Xu HR, Li H, Chen WL, Yuan F, Li XN
Received 30 December 2019
Accepted for publication 7 March 2020
Published 24 March 2020 Volume 2020:14 Pages 1219—1226
DOI https://doi.org/10.2147/DDDT.S243924
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 2
Editor who approved publication: Dr Anastasios Lymperopoulos
Purpose: Aprepitant is used to prevent nausea and vomiting associated with moderately and highly emetogenic chemotherapy. In this open-label, 2-period study, the safety, tolerability, and pharmacokinetics (PK) of aprepitant (EMEND®) were evaluated in healthy Chinese and Caucasian subjects.
Patients and Methods: Twelve Chinese and 12 Caucasian subjects were to receive a 125 mg single-dose of aprepitant during period 1; subsequently, after 15 days washout, only Chinese subjects were to receive the 3-day regimen in period 2. In each period, serial blood samples were collected and analyzed by a validated liquid chromatographic and mass spectrometric method to characterize aprepitant PK across both groups.
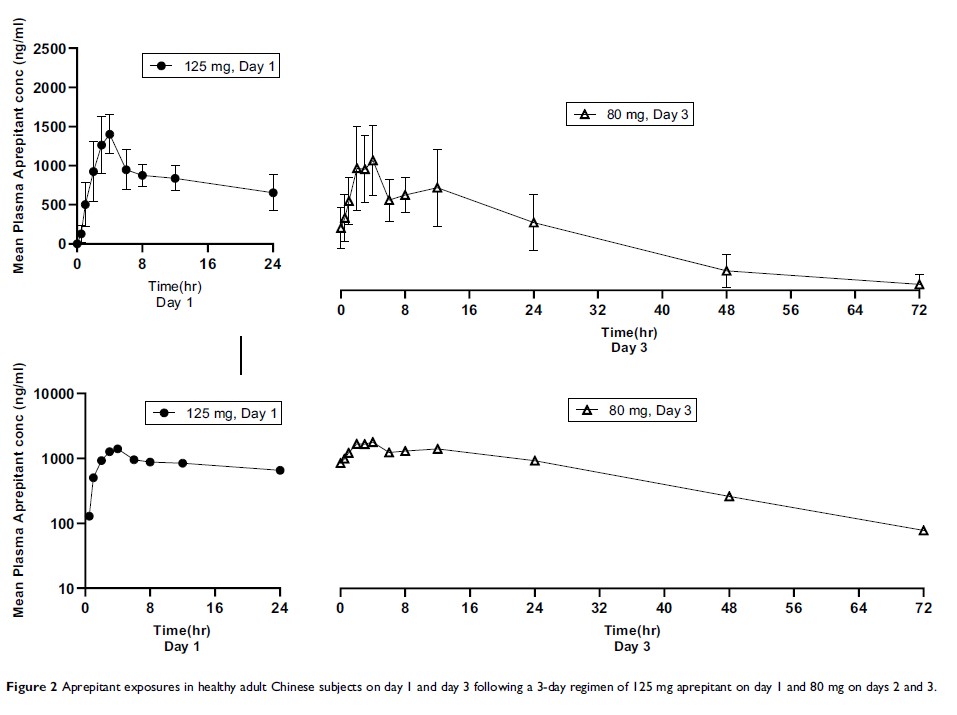
Results: In both Chinese and Caucasian subjects, there were no serious adverse events. AUC0-∞, Cmax, Tmax, and t1/2 were largely comparable between the two ethnicities. Comparing the result of period 1 in Chinese and Caucasian subjects, the geometric least-squares mean maximum plasma concentrations (Cmax) were 1482 ng/mL and 1435 ng/mL, and the area under the concentration–time curve (AUC0-∞) 34,035 hr·ng/mL and 34,188 hr·ng/mL. In period 2, the geometric mean AUC0– 24 on Day 1 and Day 3 were 19,446 hr·ng/mL and 27,843 hr·ng/mL, and the geometric mean Cmax on Day 1 and Day 3 were 1423 ng/mL and 1757 ng/mL, respectively.
Conclusion: Aprepitant is generally safe and well tolerated in healthy Chinese and Caucasian subjects. Aprepitant PK is comparable between Chinese and Caucasian subjects following single-dose administration. The PK following a clinical 3-day regimen on healthy Chinese subjects has been characterized.
Keywords: pharmacokinetics, aprepitant, chemotherapy-induced nausea and vomiting, ethnicity, healthy subjects