111314
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

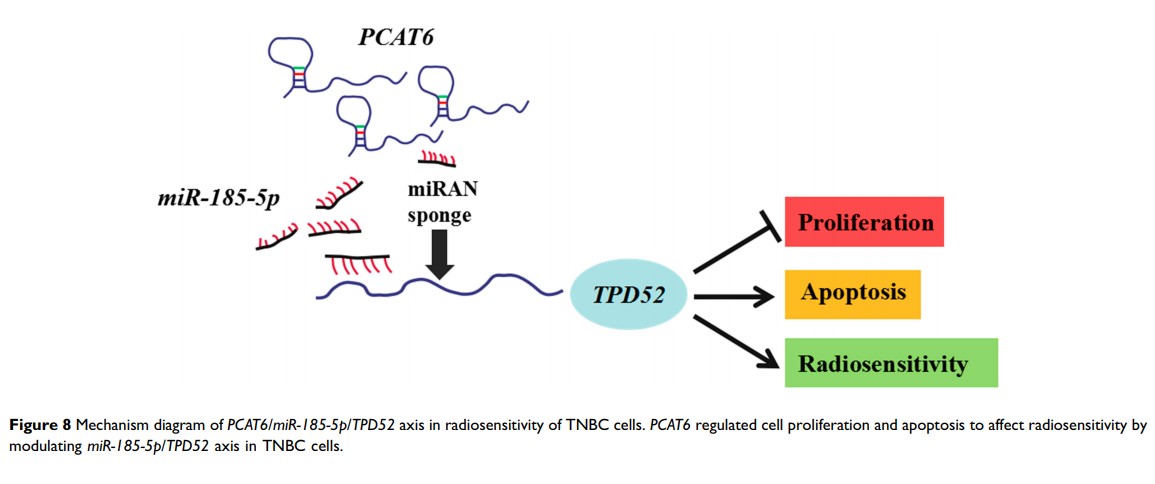
敲除 lncRNA PCAT6 可以通过调节 miR-185-5p/TPD52 轴增强三阴性乳腺癌细胞的放射敏感性
Authors Shi R, Wu P, Liu M, Chen B, Cong L
Received 6 November 2019
Accepted for publication 6 February 2020
Published 8 April 2020 Volume 2020:13 Pages 3025—3037
DOI https://doi.org/10.2147/OTT.S237559
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 2
Editor who approved publication: Dr XuYu Yang
Background: Long non-coding RNAs (lncRNAs) have been reported to play essential roles in regulating the radiosensitivity of cancers. Prostate cancer-associated transcript 6 (PCAT6 ) exerts oncogenic roles in several tumors. However, the roles of PCAT6 and its underlying mechanism in regulating the radiosensitivity of triple-negative breast cancer (TNBC) have not been investigated.
Methods: The expression levels of PCAT6, microRNA-185-5p (miR-185-5p ) and tumor protein D52 (TPD52 ) were determined by quantitative real-time polymerase chain reaction (qRT-PCR). Cell viability, apoptosis and colony formation were assessed by Cell Counting Kit-8 (CCK-8) assay, flow cytometry and colony formation assay, respectively. The interaction between miR-185-5p and PCAT6 or TPD52 was predicted by bioinformatics analysis and verified by dual-luciferase reporter assay. Western blot was carried out to detect the protein level of TPD52.
Results: PCAT6 and TPD52 were highly expressed and miR-185-5p was lowly expressed in TNBC tissues and cells, which was associated with an aggressive tumor phenotype in patients, affecting lymph node metastasis and clinical stage. PCAT6 or TPD52 knockdown or miR-185-5p overexpression enhanced the radiosensitivity of TNBC cells via inhibiting proliferation and inducing apoptosis. PCAT6 directly interacted with miR-185-5p and negatively regulated miR-185-5p expression. Moreover, TPD52 was confirmed as a target of miR-185-5p . Besides, PCAT6 regulated the radiosensitivity of TNBC cells through acting as a molecular sponge of miR-185-5p to modulate TPD52 expression.
Conclusion: Knockdown of PCAT6 promoted the radiosensitivity of TNBC cells through regulating miR-185-5p /TPD52 axis, providing a vital theoretical basis to improve the radiotherapy efficiency of TNBC.
Keywords: triple-negative breast cancer, PCAT6 , miR-185-5p , TPD52 , radiosensitivity