111314
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

RCC2 与小 GTPase RalA 相互作用并调节胃癌的细胞增殖和运动性
Authors Wang P, Zhang W, Wang L, Liang W, Cai A, Gao Y, Chen L
Received 27 August 2019
Accepted for publication 29 December 2019
Published 14 April 2020 Volume 2020:13 Pages 3093—3103
DOI https://doi.org/10.2147/OTT.S228914
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 3
Editor who approved publication: Dr Gaetano Romano
Background: Regulator of chromosome condensation 2 (RCC2), also known as TD-60, is associated with various human malignant cancers. RCC2 has been shown to exhibit guanine exchange factor (GEF) activity and contribute to early mitosis. However, the role and mechanism of RCC2 in gastric cancer remain unclear.
Materials and Methods: RCC2 expression in gastric cancer was studied using qPCR, Western blotting and immunochemistry staining of clinical specimens, and its roles in the cytobiology, mouse model and related molecular pathways were evaluated using gastric cell lines.
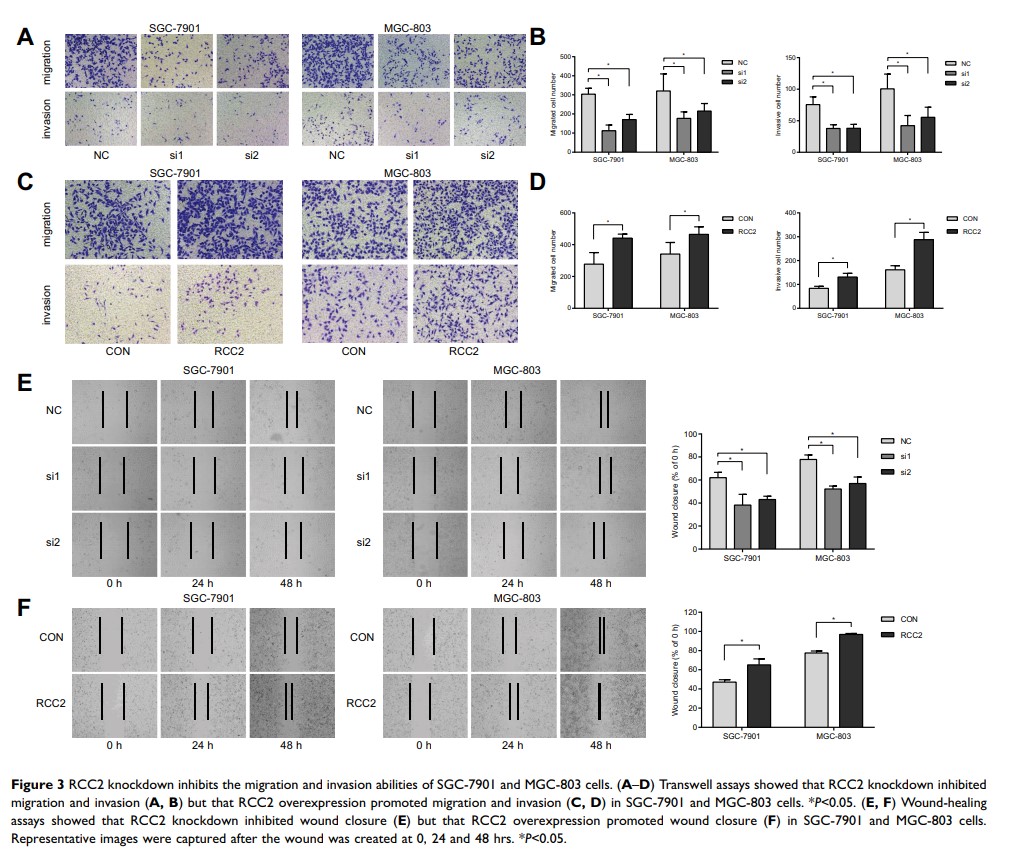
Results: RCC2 was frequently overexpressed in gastric cancer. RCC2 knockdown significantly inhibited cell proliferation, migration and invasion in vitro, which was further confirmed by the RCC2 overexpression results in gastric cancer cells. Moreover, RCC2 knockdown inhibited tumor progression in vivo. Further study revealed the interaction between RCC2 and RalA. The level of RalA-GTP was decreased in gastric cancer cells after RCC2 knockdown, while an increased phosphorylation level in MAPK/JNK was found. Furthermore, the changes in the level of RalA-GTP as well as cell proliferation, migration and invasion abilities were further confirmed using RBC8, a specific small-molecule inhibitor of the intracellular actions of Ral GTPases, in gastric cancer cells.
Conclusion: RCC2 plays an important role in gastric cancer. RCC2 knockdown inhibits cell growth, cell motility and tumor progression, which may act through RalA and affect the MAPK/JNK pathway.
Keywords: RCC2, RalA, gastric cancer, proliferation, migration and invasion