111314
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

Rap2c 作为预测脑胶质瘤预后不良的新型生物标志物
Authors Wang X, Wang C, Xi L, Yu Z
Received 30 January 2020
Accepted for publication 26 March 2020
Published 14 April 2020 Volume 2020:13 Pages 3073—3083
DOI https://doi.org/10.2147/OTT.S247731
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Takuya Aoki
Objective: Rap2c is a member of the Ras superfamily that has been implicated in various types of cancers. However, its role in glioma remains elusive. This study aimed to elucidate the role of Rap2c in glioma and its specific molecular mechanism.
Methods: We determined the expression of Rap2c in glioma tissues by Western blotting and immunohistochemistry (IHC) assays. The proliferation and apoptosis of cells were explored using CCK-8 and flow cytometry assay, whereas the migration and invasion of glioma cells were determined using transwell assay. The potential mechanism of Rap2c in the migration of glioma cell lines was investigated through Western blotting analysis and transwell assay. BALB/c nude mice were used to establish tumor models to test the effect of Rap2c on cancer metastasis in vivo.
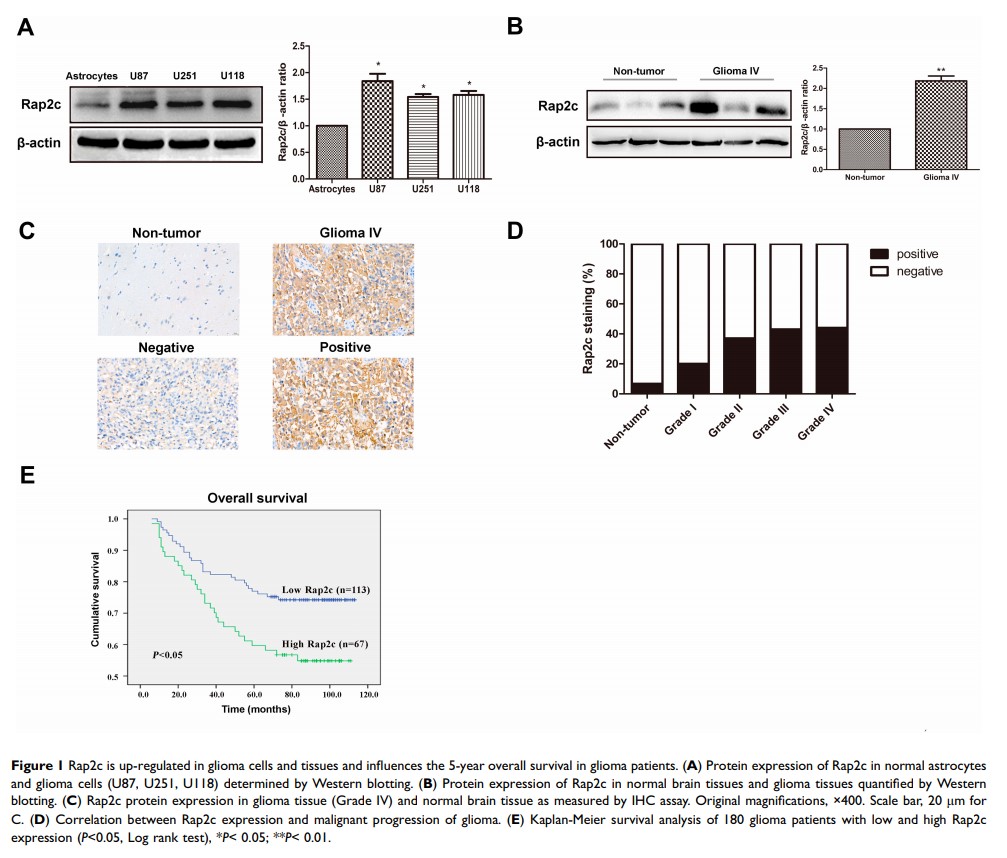
Results: Our data showed that the protein expression of Rap2c was significantly up-regulated in glioma tissues compared with normal brain tissues, and Rap2c overexpression negatively correlated with 5-year overall survival rate. However, there was no correlation between Rap2c expression and clinicopathological parameters of glioma patients. Overexpression of Rap2c promoted the migration and invasion abilities of glioma cells but had no significant effect on the proliferation of glioma cells. Western blotting analysis revealed that Rap2c overexpression increased the phosphorylation level of extracellular signal-related kinase1/2 (ERK1/2), and this effect was abolished with U0126, a selective MEK inhibitor. Furthermore, overexpression of Rap2c induced lung metastasis of glioma cells in xenograft models.
Conclusion: These findings indicate that high Rap2c expression predicts poor prognosis in glioma. Rap2c-mediated ERK1/2 phosphorylation initiates EMT cascade and promotes migration and invasion of glioma cells. Thus, targeting Rap2c and ERK signaling pathway could be a novel treatment modality for glioma.
Keywords: Rap2c, migration, invasion, MAPK, ERK, glioma