111314
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

MicroRNA-155 抑制 p38 转译并损害子宫内膜癌小鼠树突状细胞的功能
Authors Jia J, Li X, Guo S, Xie X
Received 3 December 2019
Accepted for publication 31 March 2020
Published 30 April 2020 Volume 2020:12 Pages 2993—3002
DOI https://doi.org/10.2147/CMAR.S240926
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
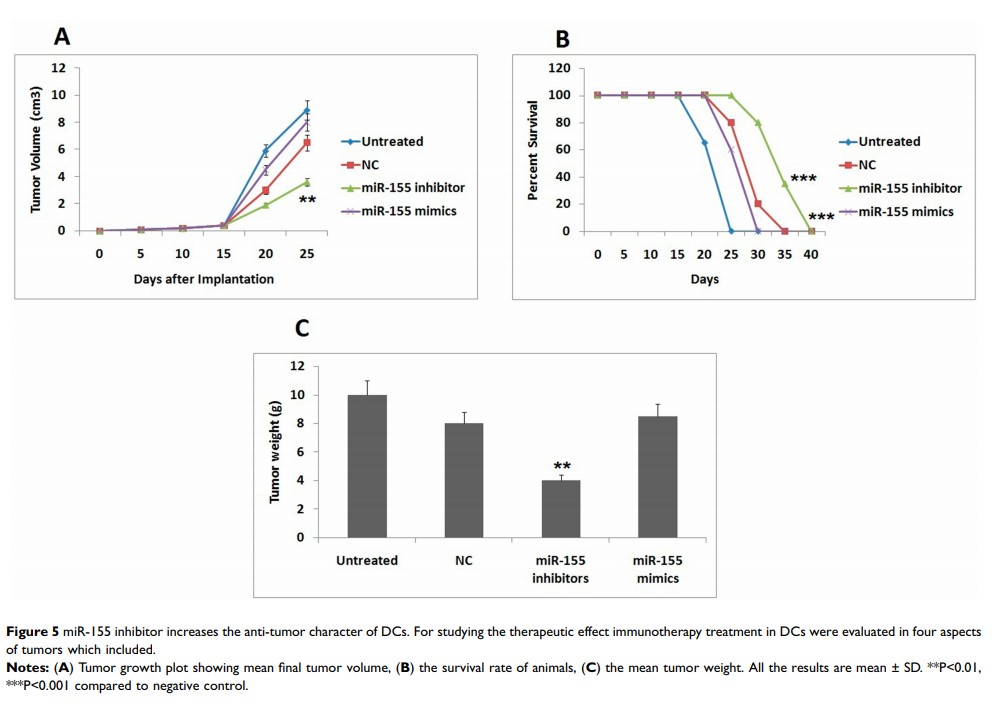
Background: Dendritic cells (DCs) are reported to play an important role in activating the anti-tumor immune responses. p38 MAPK14 signaling plays an important role in controlling their activity. Here, we identified that miR-155 suppressed the translation of p38 and impaired the functioning of dendritic cells in endometrial cancer.
Methods: HEC1A endometrial cancer cell lines were used for the study which was transfected in the C57BL/6 mice. Murine bone marrow-derived dendritic cells (BMDCs) were isolated from the mice. Target prediction was done by TargetScan which was confirmed by RT-PCR analysis. The protein expression was carried by Western blot analysis. Levels of IL-12 were evaluated by ELISA. Mice injected with HEC1A cells were subjected to tumor challenge study.
Results: On screening the binding sites of p38 MAPK14 gene, miR-155 was found to bind the 3ʹUTR directly and blocked its translation. The levels of miR-155 were upregulated in dendritic cells and RAW264.7 cells, miR-155 showed inhibitory effect on expression levels of p38. In dendritic cells, miR-155 was found to regulate the expression of IL-12, also miR-155 inhibitor stimulated the differentiation of Th1 cells in mice induced with endometrial cancer. In dendritic cells, miR-155 inhibited the expression of p38 gene and decreased their ability to interfere in tumor growth.
Conclusion: The study concludes suppressive role of miR-155 in the process of dendritic cells mediated anti-tumor immunity, also inhibiting miR-155 provides a novel strategy for countering endometrial cancer.
Keywords: miR-155, dendritic cells, p38, IL-12, RAW264.7 cells