110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

晚期肺腺癌患者与酪氨酸激酶抑制剂相关的肝毒性:一项真实世界回顾性研究
Authors Qian J, Zhang X, Zhang B, Yan B, Wang L, Gu P, Wang W, Wang H, Han B
Received 9 November 2019
Accepted for publication 2 April 2020
Published 11 May 2020 Volume 2020:12 Pages 3293—3299
DOI https://doi.org/10.2147/CMAR.S237968
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
Purpose: Hepatic injury is a common side effect following tyrosine kinase inhibitor (TKI) therapy and our understanding usually comes from clinical trials. In this retrospective study, we aimed to investigate the characteristics, risk factors and regimen-related differences of epidermal growth factor receptor (EGFR)-TKI-related hepatic toxicity in patients with advanced lung adenocarcinoma (LAD).
Patients and Methods: Liver function tests were documented in 424 patients admitted into the Shanghai Chest Hospital between January 2014 and December 2016 with advanced (IIIB/IV) LAD who received first-line gefitinib, erlotinib or icotinib. Hepatotoxicity was graded according to the Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. The clinical spectrum and onset time of hepatic injury were evaluated. The risk factors of hepatic dysfunction were determined using a logistic regression analysis.
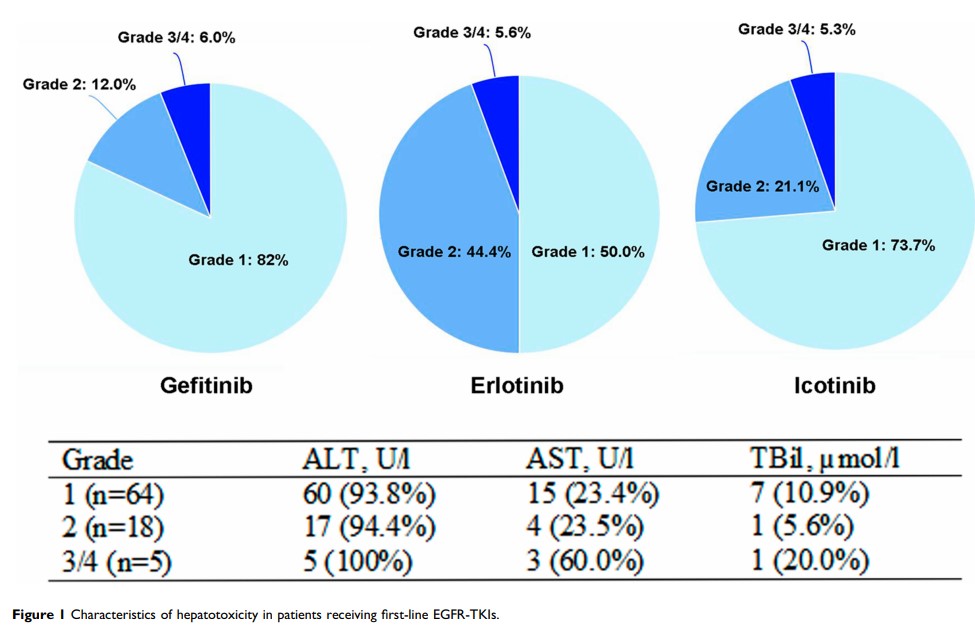
Results: A total of 87 (20.5%) patients experienced hepatotoxicity and 5.7% were of grade 3/4 liver dysfunction. The median onset time of hepatotoxicity was 7 weeks. Presence of hepatitis virus (HR: 2.593, 95% CI: 1.090– 6.170, P=0.031) and pretreatment liver impairment (HR: 3.460, 95% CI: 1.746– 6.855, P< 0.001) were risk factors associated with increased risk of hepatotoxicity. Gefitinib (HR: 1.872, 95% CI: 1.028– 3.412, P=0.040) and erlotinib (HR: 3.578, 95% CI: 1.683– 7.609, P=0.001) had increased risk of hepatotoxicity compared to icotinib.
Conclusion: The different toxic profile of EGFR-TKIs should be taken into account in the choice of treatment based on the patients’ comorbidity.
Keywords: lung adenocarcinoma, hepatotoxicity, tyrosine kinase inhibitor, gefitinib, erlotinib, icotinib