110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

布鲁顿氏酪氨酸激酶(BTK)抑制剂(依鲁替尼)抑制前列腺癌的迁移和侵袭
Authors Zhu Z, Ling L, Qi L, Chong Y, Xue L
Received 13 January 2020
Accepted for publication 14 April 2020
Published 13 May 2020 Volume 2020:13 Pages 4113—4122
DOI https://doi.org/10.2147/OTT.S245848
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 3
Editor who approved publication: Dr Sanjeev Srivastava
Introduction: Bruton’s tyrosine kinase (BTK) inhibitors have long been known in the treatment of B-cell malignancies. Recently, BTK inhibitors have also become promising novel treatment reagents for prostate cancer. The current study was designed to investigate expression of BTK in prostate cancer tissues in comparison with benign hyperplasia and effect of BTK inhibitor on prostate cancer cell proliferation, migration and invasion.
Methods: BTK expression was assessed by immunohistochemistry; migration and invasion prostate cancer cell lines (DU145 and PC3) were assessed by Transwell migration and wound-healing assay; cancer cell proliferation was assessed using MTT assay kit; expression of matrix metalloproteinases-2 and -9 (MMP-2 and MMP-9) was assessed by immunoblotting.
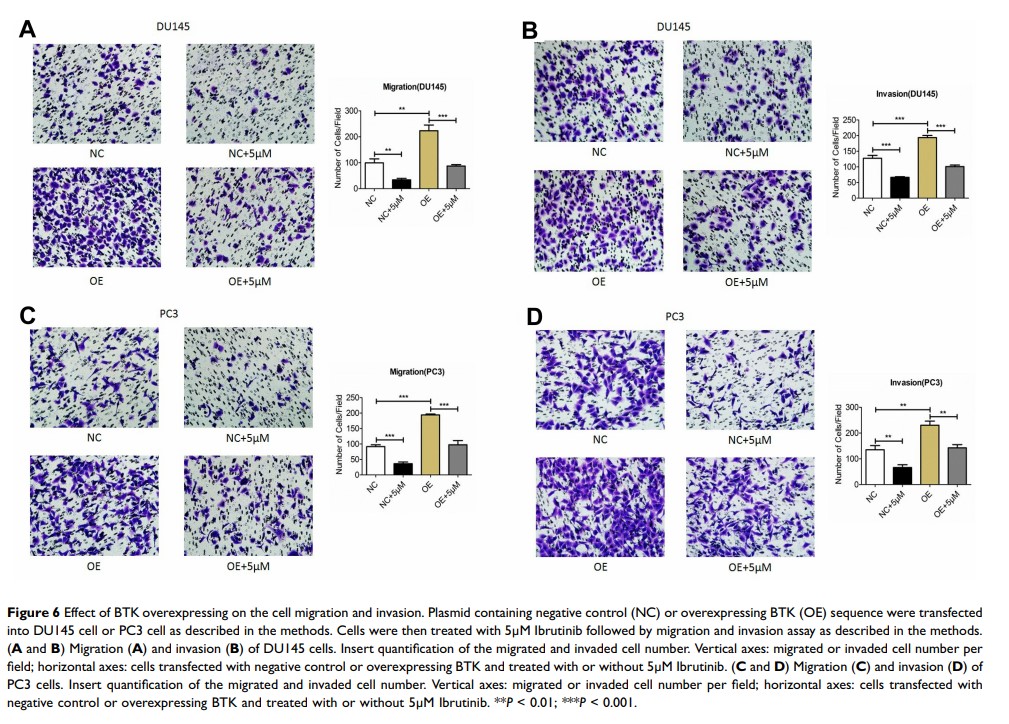
Results: Strong expression of BTK was detected in the prostate cancer tissues, especially in the tumors from prostate cancer patients with bone metastasis. BTK inhibitor (Ibrutinib) significantly inhibited cell proliferation, migration and invasion of prostate cancer cells as well as protein synthesis of MMP-2 and MMP-9 by the tumor cells. Overexpressing BTK could partially but significantly block the inhibitory effect of Ibrutinib on cell proliferation, migration and invasion, and protein synthesis of MMP-2 and MMP-9 of the cancer cells.
Conclusion: These findings suggested that BTK could serve as not only a biomarker but also a therapeutic target for the prostate cancer and that Ibrutinib may be applied as a therapeutic drug for the prostate cancer.
Keywords: Bruton’s tyrosine kinase, BTK, prostate cancer, matrix metalloproteinase, MMP