110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

淀粉酶保护的 Ag 纳米点用于体内荧光成像和肿瘤的光动力治疗
Authors Wen S, Wang W, Liu R, He P
Received 2 October 2019
Accepted for publication 21 February 2020
Published 14 May 2020 Volume 2020:15 Pages 3405—3414
DOI https://doi.org/10.2147/IJN.S233214
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 2
Editor who approved publication: Dr Linlin Sun
Background: Fluorescent metallic nanodots (NDs) have become a promising nanoprobe for a wide range of biomedical applications. Because Ag NDs have a high tendency to be oxidized, their synthesis and storage are a big challenge. Thus, the method for preparing stable Ag NDs is urgently needed. Surface modification and functionalization can enrich the capability of Ag NDs.
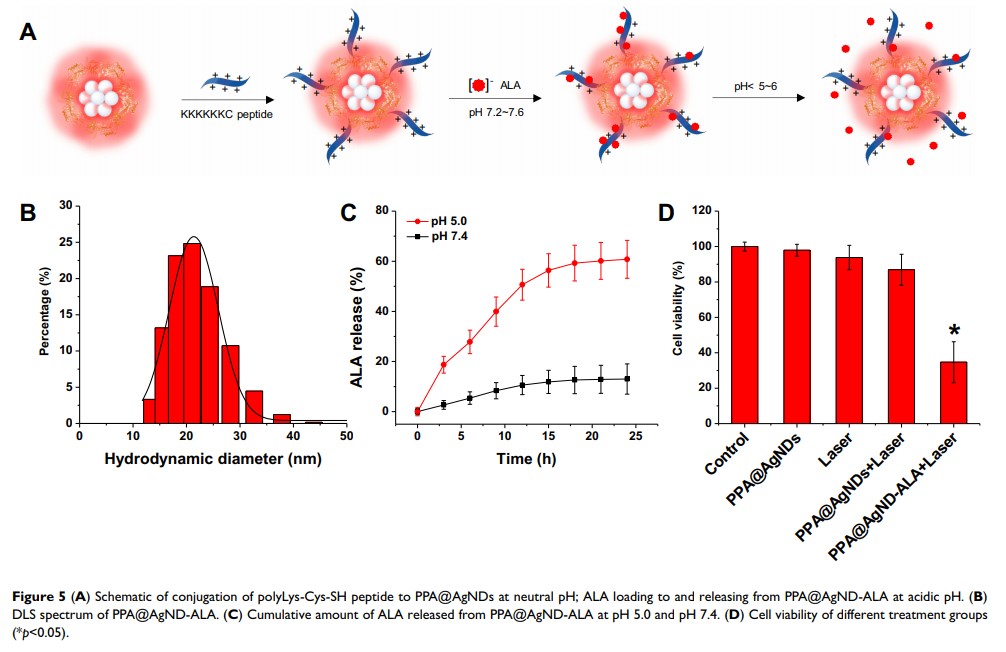
Methods: In this work, fluorescent Ag NDs were synthesized in deoxygenated water by using porcine pancreatic α-amylase (PPA) as the stabilizing/capping agent. The absorption and fluorescence of PPA-protected Ag NDs (PPA@AgNDs) were measured with a spectrophotometer and a spectrofluorometer, respectively. The morphology of PPA@AgNDs was characterized by high-angle annular dark-field (HAADF) scanning transmission electron microscopy (STEM). The biocompatibility of PPA@AgNDs was evaluated by tetrazolium (MTT)-based assay. PolyLys-Cys-SH (sequence: KKKKKKC) peptides were conjugated to PPA@AgNDs via heterobifunctional crosslinkers. PolyLys-Cys-linked PPA@AgNDs absorbed 5-aminolevulinic acid (ALA) by electrostatic interaction at physiological pH. The capability of tumor targeting was evaluated by intravenously injecting PPA@AgND-ALA into 4T1 breast cancer xenograft mouse models. Photodynamic therapy (PDT) against tumors was performed under 635 nm laser irradiation.
Results: PPA@AgNDs emitted at 640 nm with quantum yield of 2.1%. The Ag NDs exhibited strong photostability over a long period and a fluorescence lifetime of 5.1 ns. PPA@AgNDs easily entered the cells to stain the nuclei, showing the capabilities of living cell imaging with negligible cytotoxicity. ALA-loaded PPA@AgNDs (PPA@AgND-ALA) presented the superiority of passive tumor targeting via the enhanced permeability and retention (EPR) effect. Tumors were visualized in the near-infrared (NIR) region with reduced background noise. ALA molecules released from PPA@AgND-ALA was converted into the photosensitizer (PS) of protoporphyrin IX (PpIX) intracellularly and intratumorally, which greatly improved the PDT efficacy.
Conclusion: Our approach opens a new way to design a novel theranostic nanoplatform of PPA@AgND-ALA for effective tumor targeting and fluorescence image-guided PDT.
Keywords: α-amylase, Ag nanodots, peptides, targeted imaging, photodynamic therapy