110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

miR-665 通过靶向 CRIM1 抑制胃癌的上皮-间质转化和进展
Authors Wu KZ, Zhang CD, Zhang C, Pei JP, Dai DQ
Received 11 December 2019
Accepted for publication 21 April 2020
Published 15 May 2020 Volume 2020:12 Pages 3489—3501
DOI https://doi.org/10.2147/CMAR.S241795
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 2
Editor who approved publication: Dr Seema Singh
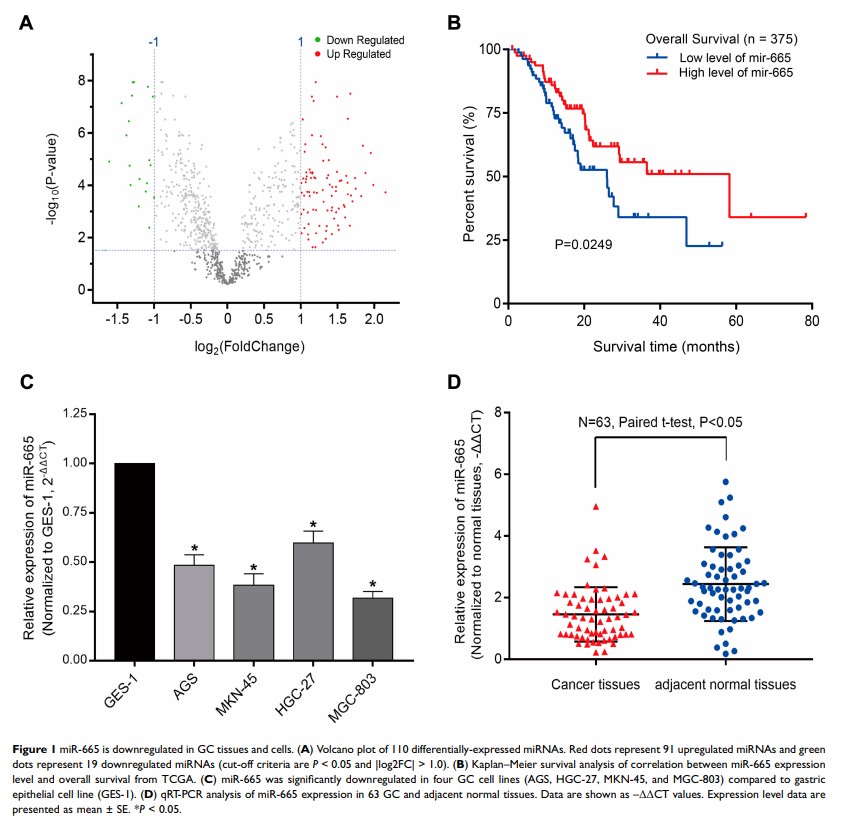
Background: Gastric cancer (GC) is one of the most common aggressive cancers and is characterized by high mortality. Increasing evidence has shown that microRNA-665 (miRNA-665) serves as inhibiting-miRNA in cancers. However, the role of miR-665 in GC is yet unclear.
Methods: miR-665 was first analyzed using bioinformatics. Subsequent quantitative real-time PCR was used to detect miR-665 expression levels in different GC cell lines and tissues. The function of miR-665 in GC cells was determined via Cell Counting Kit 8, colony formation, wound healing, and transwell assays. Furthermore, Western blotting was utilized to measure the expression level of epithelial–mesenchymal transition (EMT)-related proteins. The target prediction and luciferase reporter assays were performed to confirm the binding between miR-665 and 3ʹ-UTR of the CRIM1 gene. In addition, rescue assays were used to determine whether CRIM1 upregulation abolished the inhibitory effect of miR-665.
Results: The expression of miR-665 was significantly decreased in GC patients and GC cell lines. Clinical and pathological analyses showed that the low expression of miR-665 was significantly associated with high TNM stage (P = 0.007), distant metastasis (P = 0.031), and poor differentiation (P = 0.029). Endogenic mimics of miR-665 remarkably suppressed GC cell proliferation, migration, invasion, and EMT in in vitro experiments. Inhibition of miR-665 expression induced the opposite effects. The results of the bioinformatics analysis and dual-luciferase assay showed that miR-665 targeted the 3ʹ-UTR of the CRIM1 gene. Rescue assays revealed that overexpression of CRIM1 attenuated the inhibitory effects of miR-665 in GC progression and EMT.
Conclusion: The overall study results demonstrated that miR-665 inhibits tumor progression and EMT in GC by targeting CRIM1 , indicating that miR-665 might be a potential therapeutic target in the treatment of GC patients.
Keywords: gastric cancer, prognosis, miR-665, CRIM1 , epithelial–mesenchymal transition