110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

阿帕替尼对晚期或转移性胃腺癌或食管胃结合部腺癌患者的有效性和安全性:一项前瞻性观察研究
Authors Shen B, Jiang H, Wang L, Qian J, Shu Y, Chen P, Mao G, Liu B, Zhang X, Liu C, Wu J, Li X, Cai W, Shen W, Wang Q, He J, Hua D, Zhang Z, Zhang Y, Feng J
Received 24 September 2019
Accepted for publication 10 April 2020
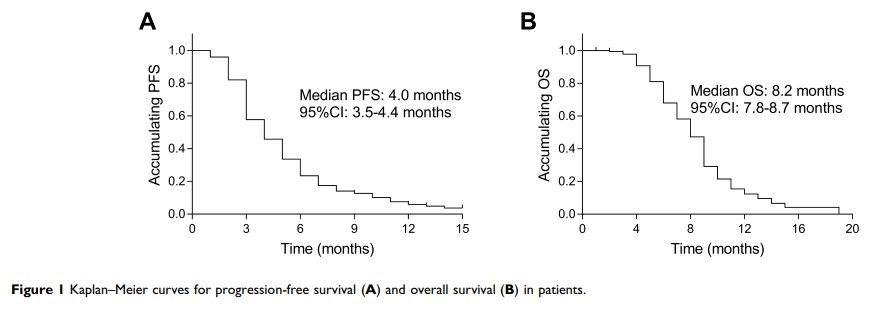
Published 20 May 2020 Volume 2020:13 Pages 4457—4464
DOI https://doi.org/10.2147/OTT.S232287
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 2
Editor who approved publication: Dr Tohru Yamada
Background: Apatinib showed promising efficacy in the treatment of advanced or metastatic gastric cancer (mGC) in previous clinical studies. However, the real-world data are limited, and this study aimed to assess the effectiveness and safety of apatinib for the treatment of advanced or mGC in this setting.
Methods: In this prospective observational study, progression-free survival (PFS), overall survival (OS), overall response rate (ORR), disease control rate (DCR) and treatment-related adverse events (AEs) were recorded and evaluated. Univariate and multivariate analyses were conducted to explore potential biomarkers which might be related to the effectiveness.
Results: A total of 321 mGC patients from 47 centers in China were enrolled between July 1, 2015, and March 1, 2018. Thirty-two patients achieved partial response, 155 patients achieved stable disease, and 115 patients had progressive disease, and no CR was achieved, illustrating an ORR of 10.60% and a DCR of 61.92%. The median PFS and OS were 4.0 and 8.2 months, respectively. Multivariate Cox analysis showed that the potential biomarkers associated with longer PFS were combination regimens plus taxel/docetaxel, and apatinib initial dosage ≥ 500mg, occurrence of AEs of leukopenia, and hand-foot syndrome. Main AEs were proteinuria (17.1%), hypertension (15.9%), and handfoot syndrome (8.7%).
Conclusion: The present prospective observational study showed favorable effectiveness and safety of apatinib in real-world patients with advanced or metastatic GC in China. (A prospective, multi-center, non-intervention study of apatinib in the treatment of advanced gastric cancer-Trial Registry Number: ChiCTR-OPN-15006601).
Keywords: apatinib, gastric cancer, non-intervention, VEGFR2 inhibitor